CRYSTAL STRUCTURE OF AN AMINO-ACYLATION CATALYTIC ANTIBODY. Ben Spiller*, B.D. Santarsiero, Linda Hsieh, Raymond Stevens, Department of Molecular and Cell Biology, University of California, Berkeley CA 94720 USA
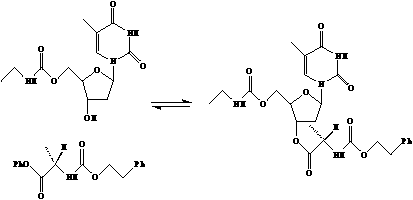
Many hydrolytic catalytic antibodies have made by raising antibodies against phosphate esters. Bimolecular addition reactions go through the same transition states as hydrolysis reactions and, with appropriate leaving groups, can be catalyzed by antibodies raised against phosphate esters.
Here, the first high resolution crystal structure of an antibody that catalyzes an addition reaction, aminoacylation, is presented. This antibody catalyzes the reaction shown. The antibody was generated by immunization with a transition state analog in which the reactive carbon ester is replaced by a phosphate ester with a phenol leaving group.

The FAB fragment was crystallized in space group P43212 with cell parameters a=60, c= 281. Data were collected on an RaxisII and the structure was determined to 2.6 Angstroms by molecular replacement.
The aminoacylation catalytic antibody is amongst the fastest catalytic antibodies, with Kcat /Km equal to 5.4 x 104 M-1 min -1(the uncatalyzed rate is 2.6 x 10-4 M-1 min-1). Remarkably, the antibody binds hapten with a Kd of 240 pM while Km's for acyl acceptor and donor are 770uM and 260uM respectively. Thus the transition state analog is bound six orders of magnitude more tightly than the ground state. The antibody efficiently transfers an acyl group to an alcohol in aqueous solution.