THE CRYSTAL STRUCTURES OF SEVERAL 48G7 CATALYTIC ANTIBODIES WITH ESTEROLYTIC ACTIVITY. Gary J. Wedemayer*, Leo Wang & Ray C. Stevens, Chemistry Department, University of California, Berkeley 94720 USA
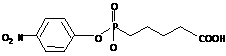
Catalytic antibodies (CA's) are immunological proteins which have been selected to catalyze specific chemical reactions. The CA 48G7, which was raised against the para-nitrophenyl phosphonate transition state shown below,

catalyzes the hydrolysis of the corresponding para- nitrophenyl ester and carbonate with acceleration rates in excess of 104 (Patten et al., Science (in press) 1996). Working with E. coli expressed protein, we have studied affinity-matured and germline antibodies. X-ray diffraction data was collected on Fab-only and on Fab-hapten crystals. Processing of this data led to four crystal structures, the best of which had a resolution of 1.95 Å. A picture of both 48G7 hapten binding and affinity maturation has emerged from this study. Of particular interest is that of the nine replacement mutations fixed during affinity maturation of this CA, none is in the active site in contact with the transition state analog. This suggests that the 104-fold increase in hapten binding seen in the mature antibody as compared to the germline results not from direct substitutions at the active site but rather from more distant replacements which alter active site geometry or conformational flexibility. Also of interest is the lack of active site serines, which are reported to play important mechanistic roles in the catalysis of other esterolytic antibodies (Zhou et al., Science 265, 1059). Instead, catalysis in the 48G7 CA appears to proceed through activated waters which are bound in the active site.