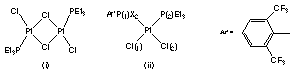
CRYSTALLOGRAPHIC STUDIES OF PHOSPHORUS CIS-PLATIN ANALOGUES WITH POSSIBLE LINKS TO ANTI-CANCER ACTIVITY. Mark. D. Roden, Dr Keith. B. Dillon, Professor Judith. A. K. Howard, University of Durham, England
A series of phosphorus (III) compounds, RPX2, (X= H, F, Cl, Br, I) and some low co-ordinate phosphorus (III) compounds have been reacted with a platinum complex (i) to form cis-platin analogues (ii). These compounds have been shown to exhibit anti-cancer activity. The rate at which compounds hydrolyse in an aqueous media is very important when considering them to be practical in vivo anti-tumour agents. The relationship between the rate of hydrolysis of the Pt-Cl bond and its bond length will be described.
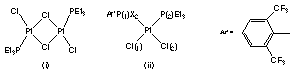
Crystallographic studies of these compounds have revealed that the Pt-Cl(2) bond trans to P(1) is significantly shorter than the cis Pt-Cl(1) bond. We believe that this variation can be correlated with the strength of the Pt-P(1) bond, which is in turn governed by the electronegativity of the substituents X, attached to P(1). Therefore by synthesising analogues with varying X substituents we hope to engineer a compound with Pt-Cl bond lengths with the optimum strength for in vivo pharmaceutical action.