STRUCTURES OF NOVEL METAL-CARBENE COMPLEXES DERIVED FROM HYPERVALENT DIAZA-DISELENATHIAPENTA LENES. F. Iwasaki, H. Nishiyama, N. Manabe, M. Yasui, N. Matsumura+, Dept. of Applied Phys. and Chem., The Univ. of Electro-Comm., Chofu, Tokyo 182, Japan, +Dept. of Applied Chem., College of Eng., Univ. of Osaka Prefecture, Sakai, Osaka 591, Japan
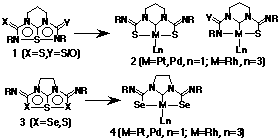
Novel metal-carbene complexes (2) with a metallapentalene framework were obtained from 6a-thiatetraazapentalenes (1) which contain a hypervalent N-S-N bond by treating with Pt(PPh3)4, Pd(PPh3)4 and Rh(PPh3)3Cl [Matsumura, et al. (1995) J. Am. Chem. Soc. 117, 3623]. X-Ray investigations revealed that the central sulfur atom in 1 was substituted by a metal atom and that thioamide groups on one or both sides were rotated to form metal-sulfur bonds in the resultant metallapentalene framework. From the MO calculations on Frontier electron densities of the tetraazathiapentalenes, 1, and the results of the structures of carbene complexes, we proposed the mechanism of the formation of these metal-carbene complexes. This mechanism suggests that starting compounds could be S-S-S or Se-S-Se hypervalent systems instead of N-S-N hypervalent systems. In fact, recently we obtained metal complexes from diselenathiapentalenes, 3. X-ray structure analyses revealed that these complexes were also novel metal-carbene complexes 4 coordinated by Se atoms. In this presentation, the structural features of diaza-tri-thia/selena-pentalenes (3) and metal-carbene complexes (4), as well as those of 2 and the reaction mechanism will be reported.
