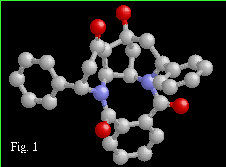
 The crystal structure exhibits a network of O-H...H hydrogen
bonds which form an infinite chain along the [0 1 0] direction
in the crystal. There are also a number of close C-H...O contacts
in the structure.
The crystal structure exhibits a network of O-H...H hydrogen
bonds which form an infinite chain along the [0 1 0] direction
in the crystal. There are also a number of close C-H...O contacts
in the structure.
 The crystal structure exhibits a network of O-H...H hydrogen
bonds which form an infinite chain along the [0 1 0] direction
in the crystal. There are also a number of close C-H...O contacts
in the structure.
The crystal structure exhibits a network of O-H...H hydrogen
bonds which form an infinite chain along the [0 1 0] direction
in the crystal. There are also a number of close C-H...O contacts
in the structure.
Another interesting feature of this structure is the presence of void space in the lattice. The total void volume in the unit cell is 33.4 Å^3 distributed over 4 symmetry equivalent sites. The difference Fourier map is featureless in the void region of the cell.
This poster will present the structure of the this novel tetracyclic compound, an analysis of the hydrogen bonding, and the geometry around the lattice voids. Crystal Data: C30 H26 N2 O4, orthorhombic, P 21 21 21, a = 8.9058(7) Å, b = 15.2549(9) Å, c = 17.455(1) Å V = 2371.4(3) Å^3, Z = 4, R = 0.050, Rw = 0.053