MORPHOLOGY OF THE EXPLOSIVE COMPOUND RDX.
J.H. ter Horst, R.M. Geertman, A.E. van der Heijden*, G.M. van Rosmalen. Delft University of Technology, Laboratory for Process Equipment, Leeghwaterstraat 44, 2628 CA Delft, The Netherlands; *TNO Prins Maurits Laboratory, Pyrotechnics and Energetic Materials, Post office box 45, 2280 AA Rijswijk, The Netherlands
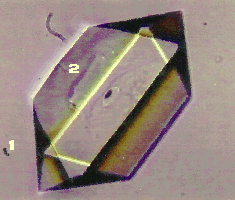
Small scale cooling crystallization experiments in stagnant media show (figure 1 and 2) that the solvent has a strong influence on the crystal morphology of RDX (cyclotrimethylene trinitramine). It is the objective of this study to find an explanation for this behavior.
A PBC (Periodic Bond Chain) Analysis is carried out in order to determine the crystal forms that may contribute to the crystal morphology. Furthermore the growth rates of all geometrically possible faces are calculated by assuming that they are proportional to the attachment energies of these faces. The combination of the PBC analysis and the attachment energy calculations results in an RDX crystal morphology prediction neglecting the influence of the solvent (figure 3). All forms observed experimentally are also present on the calculated morphology. This calculated morphology gives information about the surface structure on a molecular level and allows an estimation of the influence of the solvents on the crystal morphology e.g. by means of the sorption module of the computer program Cerius2.

Figure 1: RDX crystal grown from butyrolactone.
Figure 2: RDX crystal grown from cyclohexanone.
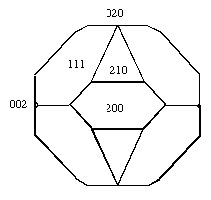
Figure 3: Calculated RDX crystal morphology.