S0174
STRUCTURE OF AMMONIUM HYDROGEN SUCCINATE AT LOW TEMPERATURES. S.
Kashino, T. Yoshida, Y. Kubozono, T. Urakawa, Y. Yoshida, H. Ishida, H. Maeda,
Faculty of Science, Okayama University, Tsushima, Okayama 700, Japan
Crystals of ammonium hydrogen succinate undergo the second order phase
transition around 170 K, and the space group is P
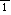 with Z=2 at 293 K (Haussühl & Schreuer, 1993). We report the
structural change accompaning the phase transition based on the structures
determined at temperatures from 297 to 20 K. The space group P
with Z=2 at 293 K (Haussühl & Schreuer, 1993). We report the
structural change accompaning the phase transition based on the structures
determined at temperatures from 297 to 20 K. The space group P
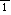 is retained, and hydrogen atoms involved in two O--H...0 hydrogen bonds between
hydrogen succinate ions are disordered at all temperatures. Temperature
dependency of the structure is remarkable in the O...O lengths of these
hydrogen bonds. These lengths decrease with a decrease in temperature from room
temperature to the phase-trasition temperature, but increase after the phase
transition. It is worthy of note that peak resolution of the difference Fourier
maps in regions of these hydrogen bonds becomes lower at 80 and 20 K
than at 150 K, suggesting some fluctuations in hydrogen- atom positions.
is retained, and hydrogen atoms involved in two O--H...0 hydrogen bonds between
hydrogen succinate ions are disordered at all temperatures. Temperature
dependency of the structure is remarkable in the O...O lengths of these
hydrogen bonds. These lengths decrease with a decrease in temperature from room
temperature to the phase-trasition temperature, but increase after the phase
transition. It is worthy of note that peak resolution of the difference Fourier
maps in regions of these hydrogen bonds becomes lower at 80 and 20 K
than at 150 K, suggesting some fluctuations in hydrogen- atom positions.
Intensity data were measured on a Hüber off-center four-circle
diffractometer in 2 [[theta]] range of 3-78deg. by using Mo K[[alpha]] X
radiation. Final R values were 0.033 for 2720 reflections at 80 K, and 0.034
for 2819 reflections at 20 K.
Haussühl, S. G. & Schreuer, J. (1993). Z. Kristallogr. 206,
255-265.
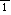 with Z=2 at 293 K (Haussühl & Schreuer, 1993). We report the
structural change accompaning the phase transition based on the structures
determined at temperatures from 297 to 20 K. The space group P
with Z=2 at 293 K (Haussühl & Schreuer, 1993). We report the
structural change accompaning the phase transition based on the structures
determined at temperatures from 297 to 20 K. The space group P
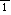 is retained, and hydrogen atoms involved in two O--H...0 hydrogen bonds between
hydrogen succinate ions are disordered at all temperatures. Temperature
dependency of the structure is remarkable in the O...O lengths of these
hydrogen bonds. These lengths decrease with a decrease in temperature from room
temperature to the phase-trasition temperature, but increase after the phase
transition. It is worthy of note that peak resolution of the difference Fourier
maps in regions of these hydrogen bonds becomes lower at 80 and 20 K
than at 150 K, suggesting some fluctuations in hydrogen- atom positions.
is retained, and hydrogen atoms involved in two O--H...0 hydrogen bonds between
hydrogen succinate ions are disordered at all temperatures. Temperature
dependency of the structure is remarkable in the O...O lengths of these
hydrogen bonds. These lengths decrease with a decrease in temperature from room
temperature to the phase-trasition temperature, but increase after the phase
transition. It is worthy of note that peak resolution of the difference Fourier
maps in regions of these hydrogen bonds becomes lower at 80 and 20 K
than at 150 K, suggesting some fluctuations in hydrogen- atom positions.