P69 PERTACTIN: LRR, PRR AND EFFICACIOUS VACCINE. Paul Emsley, Ian Charles, Neil F. Fairweather, & Neil W. Isaacs. Department of Chemistry, University of Glasgow, Glasgow G12 8QQ, UK.
Bordetella pertussis is the bacterial agent causing the respiratory disease known as whooping cough. In the course of the disease, B. pertussis binds to the pulmonary epithelial cells. One of these adhesins has been identified as pertactin. Pertactin is an Arg-Gly-Asp(RGD) sequence-containing protein. Such sequences has been known to mediate the interaction of a range of extracellular proteins to a family of cell surface adhesion receptors called integrins. The nature of the interaction of such proteins and integrins is of importance to cell attachment and mobility. Recent human trials of acellular vaccines containing pertactin show considerable efficacy (1).
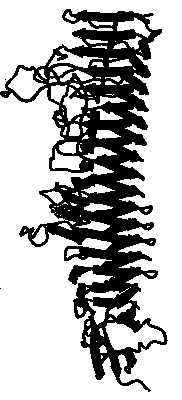
The X-ray crystal structure of pertactin has been solved to 2.5 Å using the MIRAS method. The strucuture is a 18 stranded [[beta]]-helix mostly L-shaped in cross-section(2) with 2 proline-rich regions (PRRs). The number of residues per turn varies between 18 and 25, but is typically 23. Pertactin has Leucine Rich Repeat (LRR) features, as does pectate lyase (2). There are several loops protruding from the helix. Two of these are extended proline-rich regions ((GGPXX)6) and -(PPQ)5).
The former loop is located directly after the RGD in the sequence, and is thought to mediate the interaction with epithelial cells, the latter is an immunodominant region. Pertactin may be a potential model for the structure of bacterial hemaggluttinin (FHA).
( 1 ) Science 269, 307 ( 1995).
(2) M. D. Yoder, S. E. Lietzke, and F. Jurnak, Structure 1, 241 - 251 ( 1993).
