S0397
ALIPHATIC CHAINS IN CALIXARENE DERIVATIVES E. F. Paulus, Hoechst AG,
D-65926 Frankfurt/Main; V. Böhmer, R. Arnecke, R. A. Jakobi, W.
Wasikiewicz Universität D-55099 Mainz, Germany.
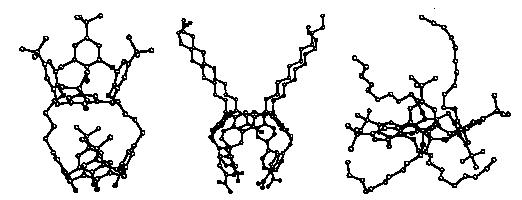
The following 3 calixarenes with aliphatic chains were investigated by single
crystal x-ray structure analysis:
In compound (I) two aliphatic chains (CH2)4O are
connecting the two calices of a couple calixarene of the head-to-tail type, in
compound (II) four aliphatic chains CH3(CH2)l0
are substituents at the 4 bridging methylene groups and in compound (III) four
of the six hydroxyl groups have the substituents
CH3(CH2)9. It can immediately be seen from the
figures, that the aliphatic chains are mostly unfolded, backfolding is
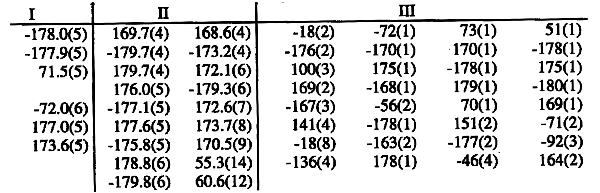
relatively seldom and occurs usually at the end of the chain. The following
table gives the corresponding torsion angles, beginning at the hydroxyl ((I),
(III)) or alkyl ((II)) end of the chain. Molecule (II) is on a special position
and has therefore only two independent chains.
 That
these conformations of but low energy, but at the same time of low entropy, can
occur, seems to be favoured by the high flexibility of the calixarenes. This
can be seen by the fact, that the calixarene molecules are on special positions
of not as high order of symmetry as is theoretically thinkable.
That
these conformations of but low energy, but at the same time of low entropy, can
occur, seems to be favoured by the high flexibility of the calixarenes. This
can be seen by the fact, that the calixarene molecules are on special positions
of not as high order of symmetry as is theoretically thinkable.
 That
these conformations of but low energy, but at the same time of low entropy, can
occur, seems to be favoured by the high flexibility of the calixarenes. This
can be seen by the fact, that the calixarene molecules are on special positions
of not as high order of symmetry as is theoretically thinkable.
That
these conformations of but low energy, but at the same time of low entropy, can
occur, seems to be favoured by the high flexibility of the calixarenes. This
can be seen by the fact, that the calixarene molecules are on special positions
of not as high order of symmetry as is theoretically thinkable.