CRYSTAL STRUCTURE AND THERMAL DECOMPOSITION STUDY OF THE [Ba2Ni(NO2)6].4H2O COMPOUND. M. Insausti, A. Bolibar, L. Lorente, J.L. Pizarro* Depts. de Química Inorgánica y Mineralogía-Petrología*. Universidad del País Vasco. Aptdo 644. 48080 Bilbao. Spain.
In order to get mixed metal oxides, the use of thermal decomposition of molecular precursors has proved to be often a more advantageous way than the ceramic method, involving lower temperatures and shorter reaction times. In this way, we have synthesized and characterized some new nitrite derivatives, and the structure of one of them, [Ba2Ni(NO2)6].4H2O, has been determined by X-ray diffraction methods
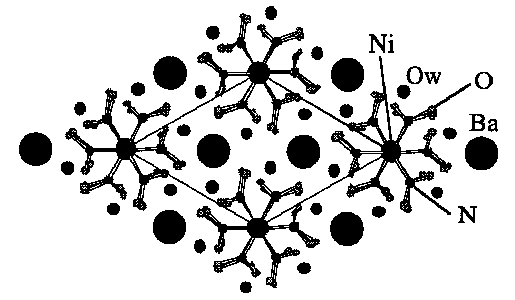
The compound crystallizes in the hexagonal space group, P63, with a = 7.525(1)Å, c = 14.516(1)Å, V = 711.8(1)Å3, Z = 2, R = 0.054, Rw = 0.066. The complex contains a three dimensional arrangement of Ni(NO2)6 entities, which are placed in the corners of the unit cell, and Ba atoms coordinated to twelve oxygens.
In order to obtain detailed information about the decomposition process, thermogravimetry and differential scanning calorymetry measurements have been performed.
