SUPRAMOLECULAR OXOMETALATE CHEMISTRY: OCTAMOLYBDATE AS A BUILDING BLOCK. Tomoji OZEKI[[daggerdbl]], Akira KITAMURA[[daggerdbl]] and Atsushi YAGASAKI[[daggerdbl]] [[daggerdbl]]Department of Chemistry, Faculty of Science, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152, Japan and [[daggerdbl]]Department of Chemistry, School of Science, Kwansei Gakuin University, Uegahara, Nishinomiya 662, Japan
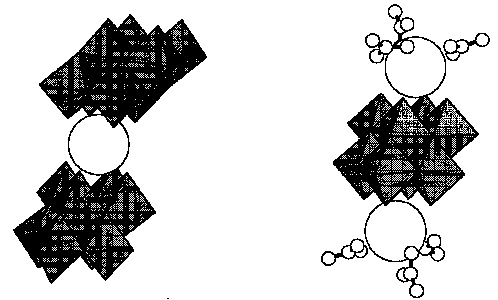
[[beta]]- Octamolybdate has been found to form two kinds of complexes with trivalent lanthanum cations: [La(MO8O26)2]5- and [La2 (NO3)6 (Mo8O26)]4-. In the former, whose analogue can also be prepared for Y, Ce, Pr, Nd, Gd and Yb, the coordination sphere of lanthanum is completed square anti-prismatically by 8 oxometalate oxygen atoms of the two [Mo8O26]4- moieties, thus forming a dimeric octamolybdate linked to each other via the La3+ cation. In the latter, nitrate anions coordinate to the La3+ cation to terminate further polymerization. So far this anion was only synthesized for La, Ce and Pr, indicating that the heavier lanthanide elements with smaller ionic radii cannot accommodate octamolybdate and nitrate anions simultaneously. These two polyoxometalate complexes show the potential to compose supramolecular oxometalate complexes utilizing octamolybdate as building blocks and the lanthanide cations as the glue.

[La(Mo8O26)2]5- [La2(NO3)6 (Mo8O26)]4-