C-Cl . . . [[pi]] (Ar) INTERACTIONS. Anne Irving, Department of Chemistry, University of Cape Town Rondebosch 770O, South Africa.
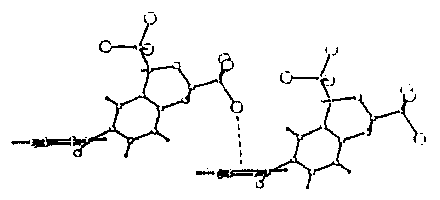
Geometry consistent with a C-Cl . . . [[pi]](Ar) interaction has been shown to exist in a wide variety ot compounds(1), (from information retrieved from the Cambridge-Structural Database(2)). An example(3) showing the geometry of a C(sp3)-Cl . . .[[pi]](Ar) interaction is given below.
The angle [C-Cl . . . [[pi]](Ar)] between the C-Cl bond and the normal to the aromatic ring varies widely, from about 90deg. to a more nearly linear arrangement.
There is some tendency for the distance of the chlorine atom from the plane of the aromatic ring to be shorter as the angle [C-Cl . . . [[pi]](Ar)] becomes closer to 180deg. .
In most compounds the C-Cl . . .[[pi]](Ar) geometry is of the 'edge' rather than the 'centroid' type ie the chlorine atom is closer to one (or two) carbon atom(s) in the aromatic ring rather than to the ring centroid.
References
1. Irving A, unpublished results.
2. Allen, F.H., Bellards, S., Brice, M.D., Cartwright, B.A.,
Doubleday, A., Higgs, H., Hummelink, T., Hummelink-Peters,
B.G., Kennard, O., Motherwell, J.R., Rodgers, J.R., and
Watson, D.G. (1976) Acta Crystallogr. B35, 2331.
3 Irving, A., and Irving, H.M.N.H. (1993) J. Cryst. Spec. Res.
23, 725.
