Next: IV. The Bauverband approach Up: Nomenclature of Inorganic Structure Types Previous: II. Coordination of the atoms
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cnom.gif)
![]()
![]()
![]()
Next: IV. The Bauverband approach
Up: Nomenclature of Inorganic Structure Types
Previous: II. Coordination of the atoms
III.1. General remarks
An acceptable nomenclature for crystal-chemical formulae should exhibit the following general characteristics:
(1) It should be as simple and self-explanatory as possible.
(2) It should retain the chemical symbols of the elements and, whenever possible, follow the normal rules of chemical formulae.
(3) It should retain other widely used symbols (e.g. coordination number, dimensionality etc.) as far as possible.
(4) It should not introduce symbols which are already widely used but with a different meaning.
(5) It should be flexible, allowing symbols to be eliminated for simplification, or permitting the inclusion of extra symbols for additional information.
(6) It should be easy to print and suitable for computer use.
The proposed nomenclature for crystal-chemical formulae is based on the
distribution of bond strengths. The spatial distribution of bond strengths in a
structure can be either homogeneous or heterogeneous. If the distribution is
heterogeneous, certain atoms![[*]](/iucr-top/logos/l2h/foot_motif.gif) are more tightly bonded together than
others, resulting in finite groups or in assemblages that are infinite in one,
two or three dimensions. These assemblages are considered as structural
units and the remaining atoms as interstitial atoms.
are more tightly bonded together than
others, resulting in finite groups or in assemblages that are infinite in one,
two or three dimensions. These assemblages are considered as structural
units and the remaining atoms as interstitial atoms.
If the spatial bond-strength distribution is homogeneous, two limiting situations may be discerned: either the structure is based on a three-dimensional framework (examples are diamond or cristobalite with directional bonds), or it is simply a packing of individual atoms (examples are helium, copper or sodium chloride with non-directional bonds). The corresponding sructural units are thus either a framework or the individual atoms, respectively.
There are five main categories of structural units, according to the kind of bond-strength distribution:
| Dimensionality of structural unit | Category of structural unit | |
| 0-dimensional | 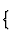 |
individual atoms |
| groups (i.e. rings, chain fragments, cages) | ||
| 1-dimensional | chains | |
| 2-dimensional | sheets | |
| 3-dimensional | frameworks. |
A structural unit may be considered to consist of subunits such as single atoms, polyhedra, single rings, single chains or single layers.
A structure can be considered to consist of structural units packed together, with interstitial atoms located between them. If the structural unit is a framework, the interstitial atoms or groups of atoms occupy holes within the framework.
Since the strengths of bonds cannot always be accurately quantified, some ambiguity may exist in assigning a structure to a given category.
III.2. Fundamental features of notation
III.2.1. General crystal-chemical formulae. Crystal-chemical formulae
give detailed structural information on the structural unit(s), their
constitution, the packing scheme, the interstitial atoms,
and the coordination of the atoms (both interstitial and those contained
in the structural units).
Symbols for atoms belonging to the structural unit(s) are placed between square
brackets, [ ], and the packing information between angle brackets,
![]() . The information on constitution which relates to the structural unit
as a whole is placed within curly brackets, { }. However, the constitutional
information which relates to subunits of the structural unit(s) may be expressed
either within curly brackets or as trailing superscripts to the chemical
elements or subunits inside the structural unit.
. The information on constitution which relates to the structural unit
as a whole is placed within curly brackets, { }. However, the constitutional
information which relates to subunits of the structural unit(s) may be expressed
either within curly brackets or as trailing superscripts to the chemical
elements or subunits inside the structural unit.
Curly brackets with constitutional information precede and angle brackets for packing information immediately follow the structural unit to which they refer.
Information concerning interstitial atoms and/or groups of atoms should generally be placed before or after that on the structural unit(s) in the sequence that chemical formulae are usually written.
In accordance with IUPAC (1990) rules, the valency state of each atom is
expressed immediately after its chemical symbol by a Roman numeral in
parentheses [e.g. Fe(III)], a superscripted Roman numeral (e.g.
Fe![]() ), or by a superscripted Arabic numeral followed by the sign + or -
(e.g. Fe3+).
), or by a superscripted Arabic numeral followed by the sign + or -
(e.g. Fe3+).
The coordination of each atom, either interstitial or in the structural unit, is
expressed within square brackets as a trailing superscript to the chemical
symbol. If additional constitutional information related to subunits is given
within the square brackets for the structural unit, then it should be placed
between Japanese quotation marks (called `brackets') below), ![]() ,
as an additional trailing superscript:
,
as an additional trailing superscript: ![]() .
.
The general notation for a compound AaBbCcDdEeFfGg could thus be:
| interstitial atoms | constitution of structural unit | structural unit | packing of structural units | interstitial atoms. |
Examples are given in ![]() III.2.2 and Table 3.
III.2.2 and Table 3.
| Compound | Crystal-chemical formulae with different structural interpretations and degrees of simplification | Bauverband description (lattice-complex notation) | |||||
| He (hex.) | He | P63/mmc | |||||
| Cu | Cu | Fm3m | |||||
| C (diamond) | C | ||||||
| NaCl | NaCl | Fm3m | |||||
| SiO2 (quartz) | SiO |
P6222 | |||||
| SiO2 (cristobalite) | SiO |
||||||
| FeS2 (pyrite) | FeS |
||||||
| FeS2 (marcasite) | FeS |
Pmnn | |||||
| (Mg,Fe)2SiO4 (olivine) | Pmcn | ||||||
| MgAl2O4 (spinel) | |||||||
| CaMgSi2O6 (diopside) | |||||||
| KAl3Si3O10(OH)2 (muscovite) | |||||||
| LaP2 (HT form) | |||||||
| Ba3AlSb3 | |||||||
| Ca3AlAs3 | |||||||
| (Mn,Fe)AlPO4(OH)2H2O (eosphorite) | |||||||
| Na3AlF6 (cryolite) | |||||||
| Ca3Si2O7 (rankinite) | |||||||
| Ca3Si2O7 (kilchoanite) | |||||||
If several distinct structural units are present, each is considered separately with its information in curly brackets followed by that in square brackets, for example:
![]()
The packing information within angle brackets describes the way the two different structural units pack together.
The hierarchy of bonds leads to a hierarchy of structural units when several degrees of bond strengths may be discerned in a structure. This often leads to weaker bond-strength units incorporating previous more strongly bonded units, and can be expressed by multiple brackets, with the central brackets referring to the structural unit having the strongest bonds:
![]()
The proposed formula can be used with any amount of any selection of structural information depending on the purpose of the study; see below.
III.2.2. Constitution of structural units. The constitution of a structural unit expresses its extensional and geometrical `structural'. i.e. the way the structural unit is built from its subunits, which may be polygons, polyhedra or any other clusters.
Some of the constitutional aspects are concerned with the structural unit as a whole, whereas other aspects are only concerned with the way each subunit is linked to other subunits. The former include dimensionality, multiplicity, branchedness and periodicity.
(i) The dimensionality is the number of dimensions in which a structural
unit has infinite extension. It is zero for individual atoms and finite groups
and one, two or three for infinite chains, sheets and frameworks, respectively.
The corresponding symbols to be used in a crystal-chemical formula are
![]() and
and ![]() .
.
The following specific symbols may be used instead of ![]() for
0-dimensional structural units:
for
0-dimensional structural units:
| individual atom: {a} | |||
| group: {g} | 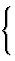
|
ring: |
|
| chain fragment: |
|
||
| cage: |
|
||
Examples are: Cs![]() S6], Na
S6], Na![]() Si4], Cu6{r}[Si6O18].6H2O.
Si4], Cu6{r}[Si6O18].6H2O.
If dimensionality is the only information expressed, the ![]() and the
pictorial symbols
and the
pictorial symbols![[*]](/iucr-top/logos/l2h/foot_motif.gif) may be used without curly brackets. Otherwise, curly
brackets are compulsory in order to avoid ambiguity.
may be used without curly brackets. Otherwise, curly
brackets are compulsory in order to avoid ambiguity.
The symbol {a} is not needed when several individual atoms, ![]() ,
considered as structural units, are written
,
considered as structural units, are written ![$[A] [B] [C]\dots$](img126.gif) . When only one atom
symbol is placed within square brackets, it means that the structural unit is
reduced to an individual atom. However, if the same atom symbols are written
[ABC], then it is necessary to add {a} in front of the square brackets.
. When only one atom
symbol is placed within square brackets, it means that the structural unit is
reduced to an individual atom. However, if the same atom symbols are written
[ABC], then it is necessary to add {a} in front of the square brackets.
In the case of group structures, e.g. ring, chain fragment, and cage structures, the number of atoms of each chemical element within square brackets must be equal to the number of atoms of each chemical element in the finite group.
(ii) The multiplicity of a structural unit is the number of single subunits, e.g. polyhedra, single rings, single chains or single layers which are linked to form a complex structural unit of the same dimensionality.
(iii) With regard to branchedness, finite structural units and single chains are called unbranched if they contain no subunits that are linked to more than two other units. They are called branched if they do. In addition, complex structural units, which can be considered as formed by linking unbranched (branched) finite structural units or single chains, are described as unbranched (branched).
(iv) The periodicity of a structural unit of infinite extension is the number of subunits, excluding branches, within one repeat unit of the chain from which the structural unit can be generated by successive linking.
For details of concepts under (ii)-(iv) see Liebau (1982, 1985); a publication on their usage in the present formulae is in preparation.
The main constitutional aspects concerned only with the way each subunit is linked to the other subunits are linkedness and connectedness.
(i) The linkedness is the number L of peripheral atoms shared between two subunits. The value of linkedness is zero for an isolated subunit. It is one or two for two subunits sharing a corner or an edge, respectively, and it is three or more for two subunits sharing a face. The average linkedness value of a subunit may be non-integral if the given subunit shares corners plus edges with different adjacent subunits.
(ii) The connectedness of a subunit is the total number s of adjacent subunits with which it shares common atoms, irrespective of its linkedness with a particular adjacent subunit. A subunit may be singular (isolated), primary (linked to only one other subunit), secondary (linked to two others), etc.
The specific values L1, L2 etc. of linkedness and/or s of connectedness of a subunit are written within `Japanese brackets' as trailing superscripts to its central atom, by analogy with the coordination symbols. The first entries in the Japanese brackets are the different values of Ln, separated from the value of s by a semicolon. The general formula for a structural unit with only one kind of subunit then reads
[Am[L1,L2,..;s]Bn].
For example, SiO2 exists in a number of polymorphs having different values of linkedness and connectedness of the SiO4 tetrahedra:
fibrous silica:
![]()
quartz, cristobalite, coesite etc. :
![]()
and stishovite
![]()
A structural unit can often be generated from a part of either lower or the same
dimensionality by a simple geometrical process that usually represents an
infinitely repeated translation. This imaginary geometrical process is called
condensation because it emphasizes the way a chain can be generated from
a group, a sheet from a chain, and a framework from a sheet. It also reveals
certain similarities between different structural units, and a specific
composite notation for the structural units has been developed which emphasizes
this interrelationship (![]() V).
V).
III.2.3. Packing of structural units. The packing of structural units expresses the three-dimensional arrangement in space. When the structural units are individual atoms, the known nomenclature for describing the packing of atoms (three-dimensional and layer-stacking descriptions) may be used. When the structural units are groups, their centres of gravity may be used with the same nomenclature as for the packing of atoms. However, this will be an incomplete description because of the lack of information on the orientation of the groups.
Packing of structural units in structures based on groups, infinite chains or sheets may be treated by layer description. Such a layer description consists of slicing the structure into layers which, by stacking, completely generate the original crystal structure. Structural units should be preserved intact in the process of slicing. The structure is then described by the packing of structural units in the layer and by a set of stacking operators.
The layer description can also be applied to framework structures taking into consideration the fact that the units operated upon are parts of a single framework.
With respect to the nomenclature for the packing of structural units, only the symbols for cubic closest packing, c, and hexagonal closest packing, h, and their sequential combination are adopted here. When no other packing information is provided these symbols may be given as trailing superscripts to the square brackets which contain the structural unit. In this case, angle brackets are not compulsory. Any other packing information, particularly the packing (or stacking) symbolism used by individual authors should be given in angle brackets on the line.
![]()
If packing information is to be given for a set of atoms which does not constitute a structural unit, the symbol should be placed within vertical bars followed by the packing information:
![]()
Copyright © 1990, 1998 International Union of Crystallography
IUCr Webmaster