Next: References Up: The Study of Metals and Alloys Previous: 8. Quaternary and Other Systems
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: References
Up: The Study of Metals and Alloys
Previous: 8. Quaternary and Other Systems
When X-ray methods were first introduced, it was hoped that they would clear up most of the problems raised by classical metallurgy. To a large extent they did so, but they exposed so many others that one eminent metallurgist said that they `raised more problems than they solved'. This was intended as a criticism but it was really a compliment; X-rays were giving us a deeper insight into the solid state.
One early important result was the identification of the structures of iron.
Cooling curves (Section 1) had shown discontinuities at 770![]() C,
910
C,
910![]() C and 1380
C and 1380![]() C and thus it was deduced that there were
four solid phases, which were called
C and thus it was deduced that there were
four solid phases, which were called ![]() (the lowest range),
(the lowest range), ![]() ,
,![]() and
and ![]() . X-ray diffraction showed however that
. X-ray diffraction showed however that ![]() and
and
![]() were identical - body-centred cubic - the discontinuity between them
being caused by the change from ferromagnetism (
were identical - body-centred cubic - the discontinuity between them
being caused by the change from ferromagnetism (![]() ) to paramagnetism
(
) to paramagnetism
(![]() ) at the Curie point. The
) at the Curie point. The ![]() phase is called ferrite,
phase is called ferrite, ![]() is
called austenite (face-centred cubic) and
is
called austenite (face-centred cubic) and ![]() is body-centred cubic again.
This work was carried out with an iron wire as a specimen, heated by an electric
current; the
is body-centred cubic again.
This work was carried out with an iron wire as a specimen, heated by an electric
current; the ![]() phase in pure iron cannot be retained by quenching
(Section 6).
phase in pure iron cannot be retained by quenching
(Section 6).
An unexpected effect was that produced by the diffusion of atoms into regular
positions - the superlattices . Most solid solutions have atoms
distributed at random on the available lattice positions, but sometimes after
annealing - or even at room temperature - the atoms distribute themselves in a
regular way. Thus an alloy of composition AuCu3 is face-centred cubic with
gold and copper atoms sharing the lattice positions at random; but during
annealing the atoms move so that no two gold atoms are neighbours and the
structure can be described as having gold atoms at cube corners and the copper
atoms at face centres. The structure is now essentially simple cubic and the
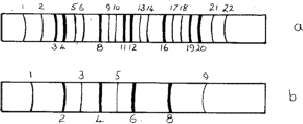
exclusion rules (Section 4.1) no longer apply. Lines 1, 2, 5, 6![]() are now
possible; these are called superlattice lines (Fig. 10a). They are
weaker than the main lines because their intensities depend upon the
difference between the scattering factors of Cu and Au.
are now
possible; these are called superlattice lines (Fig. 10a). They are
weaker than the main lines because their intensities depend upon the
difference between the scattering factors of Cu and Au.
Superlattices are also possible in body-centred structures such as NiAl, the nickel atoms being at the corners and the aluminium atoms at the centres of the unit cell. The sequence of lines is shown in Fig. 10b.
 |
Superlattice lines are sometimes much broader than the main lines, suggesting that regions over which the superlattice is perfect are quite small. This effect has been studied for over fifty years: AuCu3 is probably easily the most photogenic X-ray specimen!
When the two atoms have nearly equal atomic numbers the superlattice lines are very weak; for CuZn (29 and 30) they would be impossible to detect. To find whether such a superlattice exists, Zn radiation was used; this depresses the scattering factor of Cu and makes the superlattice lines just detectable.
Sometimes a solid solution at high temperatures will break down into two solid solutions at lower temperatures, possibly one having a superlattice; this is called a solubility gap . This effect can be detected only by X-ray methods. Even then, it is not always obvious because the two phases must have the same structure and can be detected only by their different lattice parameters. The ability to detect small lattice parameter changes by high-angle lines (Section 5) is then very important.
Sometimes, a high-temperature structure will change into a related structure of lower symmetry as the temperature falls. (It is a general rule, rarely disobeyed, that a high-temperature structure cannot be of lower symmetry than a low-temperature structure.) This, again, can often be recognized only in the high-angle lines. For example, if a cubic structure becomes tetragonal, line 16 (400) will divide into two components (400 and 004) with slightly different spacings. Thus line 16 becomes double, with one component stronger than the other because 400 and 040 have the same spacing.
Lack of equilibrium is indicated by broadened lines, and this effect must always be eliminated, if possible, by annealing in the ingot form or in the powder. The ingot may not be uniform (coring) or the powder may have strains in it. Annealing may not always be effective, presumably because the ideal equilibrium reactions take place at temperatures too low for adequate diffusion to take place. It is thought that this is the reason why the Fe-Ni system has never been established, either by classical methods or by X-ray diffraction.
There are many other problems that could be discussed, and doubtless others that have not yet been uncovered. It is therefore necessary to keep a flexible mind to cope with any new phenomena that may arise; the general principles can be described as this article has attempted to show, but there is still room for completely new ideas to emerge.
Copyright © 1984, 1998 International Union of Crystallography
IUCr Webmaster