Next: 4.1 Cubic structures Up: The Study of Metals and Alloys Previous: 3. Measurement of Debye-Scherrer Photographs
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: 4.1 Cubic structures
Up: The Study of Metals and Alloys
Previous: 3. Measurement of Debye-Scherrer Photographs
We must first have the values of ![]() for all lines on a powder photograph,
and, if the number is large, assigning indices hkl to them all may be
difficult. Fortunately, many metal and alloy structures are so simple that
their powder pattern can be recognized at a glance. Our first task is thus to
familiarize ourselves with these patterns.
for all lines on a powder photograph,
and, if the number is large, assigning indices hkl to them all may be
difficult. Fortunately, many metal and alloy structures are so simple that
their powder pattern can be recognized at a glance. Our first task is thus to
familiarize ourselves with these patterns.
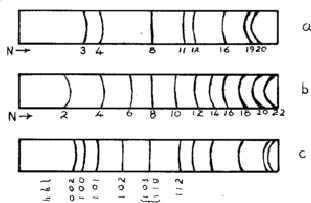
The three most common structures are called face-centred cubic (f.c.c.), body-centred cubic (b.c.c.) and hexagonal close-packed (h.c.p.). They are illustrated in Fig. 4. The sequence of lines - in position and intensity - is characteristic of each structure and the scale of the pattern gives the dimensions of the unit cell; the smaller the scale of the pattern, the larger is the unit cell. Therefore there are more lines for a larger unit cell.
 |
Copyright © 1984, 1998 International Union of Crystallography
IUCr Webmaster