Next: How Numerous are the Reliable Triplets Up: An Introduction to Direct Methods. The Previous: The Positive Quartet Relation
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: How Numerous are the Reliable Triplets
Up: An Introduction to Direct Methods. The
Previous: The Positive Quartet Relation
In the event that the sum of the four phases is equal to ![]() :
:
| (34) |
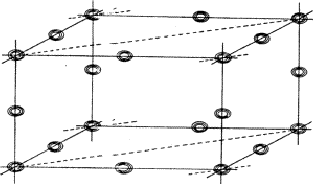
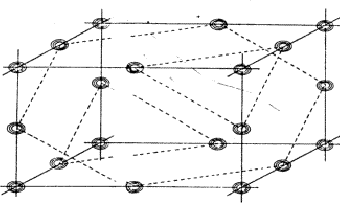
The planes of maximum for the 4 reflection involved in relation (34) are indicated in Fig. 16. It can be seen that for all indicated positions three out of four planes intersect. If atoms are located at these points the resulting unitary structure factors of H, K, L and -H-K-L will be 0.5, because three atoms lie in the planes and one lies halfway between. Thus for a negative quartet relation the reflections H, K, L and -H-K-L will in general not be found amongst the very strongest. The next question to be answered is: what is the intensity of reflection H + K, if the electron density is located near the marked points of Fig. 16? From Fig. 17 it can be easily seen that H + K will have a small |E|-magnitude: equal numbers of points of electron density concentration lie on the H + K planes and halfway in between. As the same holds for the other cross terms it can be stated that the negative quartet relation (34) is likely to be true for reasonably large values of E4 and small |EH+K|, |EH+L| and |EK+L|.
 |
 |
Copyright © 1984, 1998 International Union of Crystallography
IUCr Webmaster