 |
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
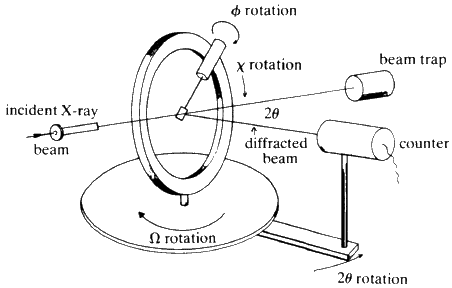
All of the methods described so far use X-ray film for recording the reflections. They may all be modified so that a Geiger counter or other electronic detector can be used to detect both the presence and intensity of each of the reflections. There are now computer-controlled diffractometers available commercially that require only that the crystal be aligned in the X-ray beam and then the instrument will in turn, measure and refine the unit cell dimensions, record all reflections out to a chosen maximum theta-angle, and punch out the values of hkl, intensity, and estimated error for each reflection. The geometry of a 4-circle instrument is shown schematically in Fig. 29.
An automatic diffractometer can easily measure reflections from a single crystal at the rate of one per minute, so the data for any normal crystal structure can be collected in a few days. With the aid of a competent crystallographer and a modern computer, the detailed molecular structure of any molecule of up to 100 atoms (not counting hydrogens) can be solved in less than three weeks. (Compare this with the months or years required by degradative chemical techniques.) All that is needed is: a good crystal, about 0.3 mm cubed, of a derivative containing one atom of atomic number round about 30. For compounds of up to 20 C, N, O, one Cl is adequate; up to 50 C, N, O, one Br is adequate (e.g. a parabromobenzoate is a very useful derivative); for about 100 C, N, O, one Iodine is acceptable, but two Br are probably better. Potassium or rubidium salts are suitable for organic acids.
Copyright © 1981, 1997 International Union of Crystallography
IUCr Webmaster