This is an archive copy of the IUCr web site dating from 2008. For current content please visit
https://www.iucr.org.




Next: Diffraction of X-rays
Up: An Introduction to the Scope, Potential
Previous: Structure of Crystals
The abstractions of unit cells, lattice and symmetry to which reference
has already been made lead to the possibility of classifying all crystals into
well defined systems. On mathematical grounds it can be shown that there
are only 7 possible unit-cell types (the 7 crystal systems) but, because
some are capable of replicating themselves in a lattice in more than one
way there are 14 possible lattices (the so-called Bravais lattices (Table 3)).
There are 32 ways in which symmetry elements that can be detected by
visual or morphological examination can be arranged (the 32 point
groups) but, if all the symmetries possible on an atomic scale are included
there are 230 possible arrangements (the 230 space groups).
It is interesting and important to remember that no crystal can have a
5-fold axis of symmetry. However, individual molecules can have such an
axis of symmetry. A crystal cannot have a 5-fold axis because there is no
way of packing by translation an infinite number of unit cells which have
a pentagonal cross section so that they completely fill space.
Because space group symmetry is of little importance in the applications
to be described, it will not be dealt with. Of course, a full understanding
of the 230 space groups is necessary for anyone undertaking the
determination of molecular structure by X-ray diffraction.
It will now be useful to consider the concept of families of planes
running through a crystal and their labelling by the use of Miller indices.
We will start by considering lines in two dimensions.
Figure 10
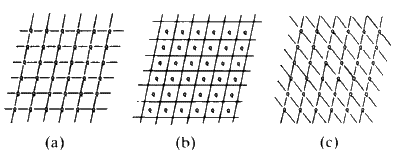 |
If we join the corners of the unit cells, or any other point in the cell, by
straight lines we generate families of lines. All the lines in a family are
parallel to each other and run through the same number of lattice points.
Figure 10 shows three examples.
Figure 11
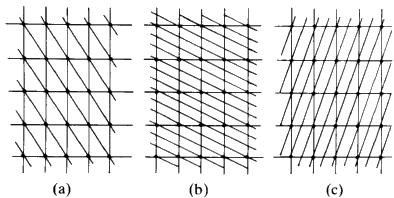 |
Consider Fig. 11(a). Through each cell there is only one plane. Start
from the lattice point at the bottom left hand corner, and consider that as
the origin. You will travel along the vertical direction one cell edge before
you encounter such a line; if you travel along the horizontal axis you will
move one cell edge before you encounter a line. Consider the set of
planes in sketch b. Start at the origin and move along the vertical axis.
You will come in contact with three lines before you reach the first lattice
point along that edge. If you move along the horizontal direction you will
come in contact with only one line before you reach the first lattice point.
In sketch c in moving along the vertical direction from the origin, to the
first lattice point, you contact only one line; moving along the horizontal
direction, you cross two lines. It should be fairly obvious that you can
describe the geometry of these lines simply in terms of how many cut one
unit cell edge or into what number of equal size pieces this family of lines
will cut one unit cell edge. In sketch a, the family cuts the vertical axis
into one and the horizontal axis into one. In sketch b, the vertical axis is
cut into three, the horizontal axis, one. In sketch c, the vertical axis is
cut once while the horizontal is cut twice. So the planes in sketch a could be
labelled 1:1; those in b could be labelled 3:1, those in c could be
labelled 1:2. The sign of the slope of the planes is obviously different
between b and c and so we will have to define the positive and negative
directions along the edges of the cell. Define the vertical direction,
starting from the bottom left hand corner, going up as positive and
horizontal, starting at the left hand corner, going across to the right as
positive. We would then find that in a, you could number these planes as
(+1 +1), in b they would be labelled (+3 +1) and in c the label would be
(+1 -2). These numbers that can be assigned to the lines are called Miller
Indices, and they are characteristic of these lines. It is obvious that the
larger the Miller Indices that we can assign to the family of lines, the
closer together these lines will be; in other words, the smaller will be the
perpendicular distance between adjacent parallel lines. It should also be
obvious that the distance between any pair of lines will be the same no
matter where you are in the crystal because all the unit cells are the same
size. What was done here in two dimensions is easily extended to three
dimensions. Thus, any plane running through a crystal and passing
through the set of lattice points--in other words through the corners of
the unit cells--can be identified by a triplet of integers; the Miller Indices.
The symbols used are the alphabetical letters (hkl). We can describe any
plane in a crystal by three integers and these numbers can have + or -
signs. A negative index, -n, is usually written as  for convenience.
Typical families of widely spaced planes might be: (110),
for convenience.
Typical families of widely spaced planes might be: (110), 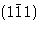 etc.:
planes such as (742),
etc.:
planes such as (742), 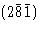 etc., would be much closer together.
etc., would be much closer together.


Next: Diffraction of X-rays
Up: An Introduction to the Scope, Potential
Previous: Structure of Crystals
Copyright © 1981, 1997 International Union of Crystallography
IUCr Webmaster



