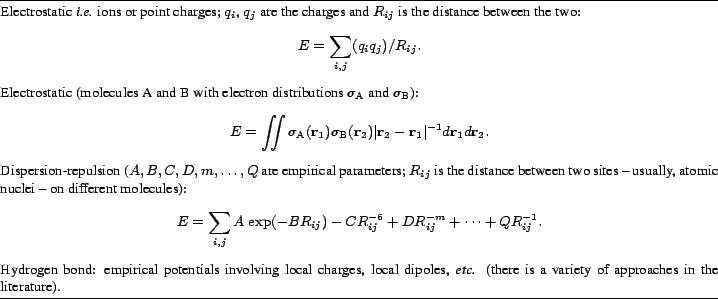 |
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
A molecule consists of a collection of positively charged atomic nuclei surrounded by an electron cloud. Even if the molecule has no net charge, such an object can hardly be considered as electrically neutral. Its electrostatic potential is a superposition of the fields of all nuclei and electrons. An approaching charge can alter, by its own electrostatic field, the electron distribution in a molecule; this phenomenon is called polarization.
The attractive forces holding molecules together are a consequence of molecular electrostatic potentials. For purely ionic crystals, one can just use Coulomb's law with integer charges; for organic molecules, it takes a more complicated expression, involving an integration over continuous electron densities. Alternatively, the charge distribution can be represented by a series expansion using multipoles, and the interaction energy can be calculated as a function of multipole moments.
Different atoms have different electronegativities. Larger charge separations within the molecule - in the jargon of the trade, more polar molecules - build up stronger intermolecular forces. Ionic crystals are very hard and stable, while naphthalene or camphor (two common ingredients of mothballs) sublime rather easily. These non-polar hydrocarbon molecules must rely on mutual polarization to produce attraction; the resulting forces are feeble, and are called dispersion or van der Waals' forces; they are usually described by empirical formulae. In this way, even argon manages to form a solid at very low temperature.
Ubiquitous in crystals is the hydrogen bond, a polar interaction which is the most effective means of recognition and attraction between molecules; so effective, that molecules with donor and acceptor groups form hydrogen bonds without exception. There is no case (at least, to the authors' knowledge) where a molecule that can form hydrogen bonds does not do so in the crystal.
The repulsion at short intermolecular distance arises from a quantum mechanical effect. According to Pauli's principle, electrons with the same quantum numbers, no matter if belonging to different molecules, cannot occupy the same region of space. Thus, Pauli "forces" - although they are not forces in the sense of Newtonian mechanics - steer electrons to mutual avoidance, and the total energy of the electron system rises if paired electrons are pulled together.
Table 1 collects the simple potentials mentioned so far. Direct but non-specific measures of the strength of crystal forces are the melting temperature and the sublimation enthalpy.
Copyright © 2005 International Union of Crystallography
IUCr Webmaster