IUCr journals news
Recent developments in microdiffraction on protein crystals
J. Synchrotron Rad. (2004). 11, 4-6 [doi:10.1107/S090904950302541X]
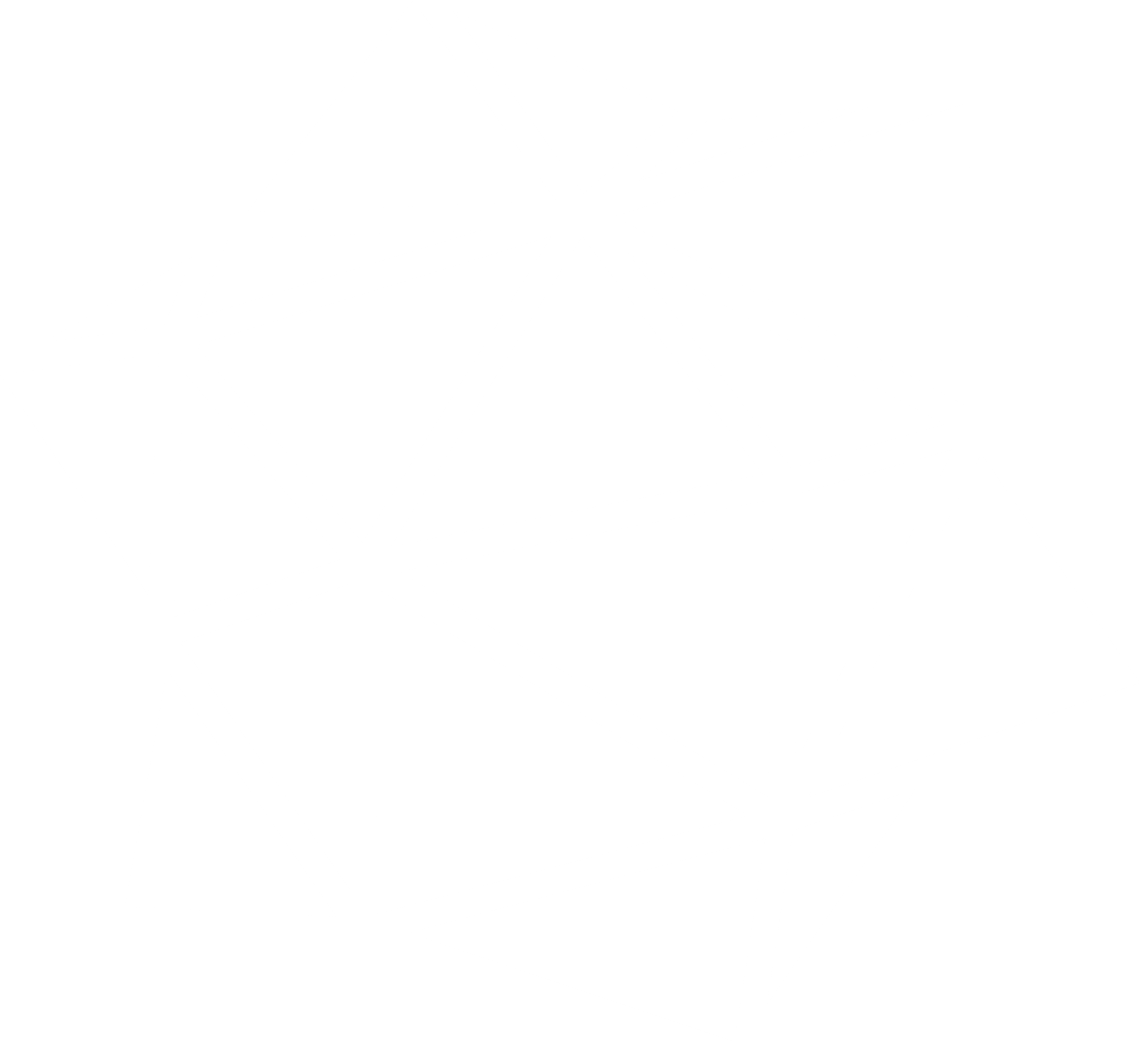
![[Bovine rhodopsin]](https://www.iucr.org/__data/assets/image/0004/10201/jsr.gif) Cryocooled crystal of bovine rhodopsin visualized on the microgoniometer. (Courtesy of G. Schertler, Cambridge)
Cryocooled crystal of bovine rhodopsin visualized on the microgoniometer. (Courtesy of G. Schertler, Cambridge)
A dedicated microgoniometer for synchrotron radiation protein crystallography has been developed at EMBL-Grenoble in collaboration with ESRF. Beam sizes down to about 5 μm and a compact pinhole collimating system allow reducing background scattering from the sample environment, which is important for small and weakly scattering crystals such as membrane proteins. A microscope, coaxial to the X-ray beam, facilitates sample alignment. Needle-like protein crystals of about 10 μm diameter of trigonal bovine rhodopsin belong to the currently smallest protein crystals studied.
C. Riekel