Fluorescence-based protein crystal identification
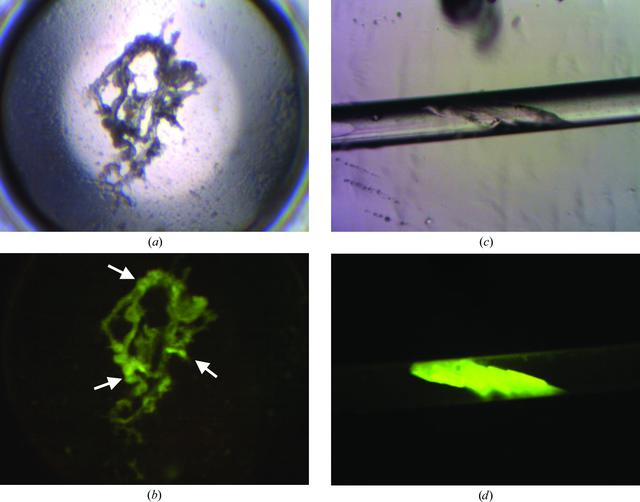 Successful protein crystallization screening experiments are dependent upon the experimenter being able to identify positive outcomes. The introduction of fluorescence techniques has brought a powerful and versatile tool to the aid of the crystal grower. Trace fluorescent labeling, in which a fluorescent probe is covalently bound to a subpopulation of the protein, enables the use of visible fluorescence. Alternatively one can avoid covalent modification and use UV fluorescence, exploiting the intrinsic fluorescent amino acids present in most proteins. By the use of these techniques, crystals that had previously been obscured in the crystallization drop can readily be identified and distinguished from amorphous precipitate or salt crystals.
Successful protein crystallization screening experiments are dependent upon the experimenter being able to identify positive outcomes. The introduction of fluorescence techniques has brought a powerful and versatile tool to the aid of the crystal grower. Trace fluorescent labeling, in which a fluorescent probe is covalently bound to a subpopulation of the protein, enables the use of visible fluorescence. Alternatively one can avoid covalent modification and use UV fluorescence, exploiting the intrinsic fluorescent amino acids present in most proteins. By the use of these techniques, crystals that had previously been obscured in the crystallization drop can readily be identified and distinguished from amorphous precipitate or salt crystals.
Overall, fluorescence, whether intrinsic or by using trace fluorescent labeling (TFL), can be a powerful aid in macromolecule crystallization. Here Meyer et al. [(2015). Acta Cryst. F71, 121-131; doi:10.1107/S2053230X15000114] have only discussed its use in screening for crystals, although other applications in the field of macromolecule crystallization and crystal growth are possible. Simple instrumentation incorporating the requisite basic functionality for the three main approaches discussed in the paper can be realized in even a small structural biology laboratory. The benefits obtained are powerful aids in interpreting the screening results as well as obtaining potential insights leading to additional, previously unrealized, lead conditions.


