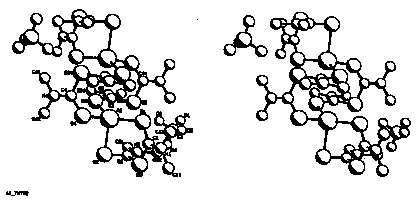
SYNTHESIS AND STRUCTURE OF Ag-TMTMS COMPLEX: (Ag-TETRAMETHYLTHIURAMMONOSULFIDE) By M. Kawaminami*, C. S. Mendoza and S. Kamata, Dept. of Phys. College of Liberal Arts* and Dept of Applied Chemistry and Chemical Engineering, Kagoshima University, Kagoshima 890, JAPAN.
The silver selectivity of TMTMS using solvent extraction is studied. A 1g AgClO4 was dissolved in a 50-ml acetone solution (90% acetone + 10% water) and mixed with a 2.5g TMTMS dissolved in a 50-ml acetone. After long standing, yellowish crystals suitable for X-ray analysis were formed, separated and washed with acetone. The data collection was made by using a CAD4 diffractometer, and the structure was solved by direct methods and refined in full-matrix least-squares. All calculations were performed on a VAX computer using MOLEN.
AgClO4(C6H24S3N2)2, Mr=624.06, monoclinic, P21/n, a=13.9249(6)Å, b=9.9342(4)Å, c=18.0702(15)Å, ,[[beta]]=108.37(1)deg., V=2372.3(2)Å3, Z=4, Dcal=1.75g/cm3, [[lambda]](Mok[[alpha]])=0.71073Å, u=14.9cm-1, [[omega]]/2[[theta]] scan, 2[[theta]]max=60.9deg., F(000)=1264, T=21deg.C, R= .043, wR= .057 for 4028 reflections.
Ag atom bonded with 4 S atoms of two molecules of the ligand forming a bidentate molecule, in which a center of symmetry are connected to each other making a ring of Agl-S-C-S-C-S-Ag2.
