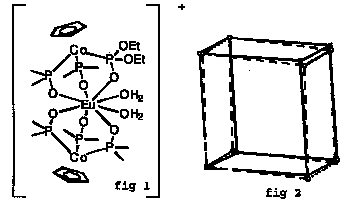
SOLID STATE CONVERSION IN SINGLE CRYSTALS OF A COORDINATION COMPOUND. Ulli Englert, Beate Ganter, and Trixie Wagner, Institut f. Anorg. Chemie, RWTH Aachen,, 52056 Aachen, Germany; Wolfgang Kläui, Institut f. Anorg. Chemie, Heinrich-Heine-Universität, 40225 Düsseldorf, Germany.
Topotactic elimination of an aqua ligand in [Eu<LOEt>2(H2O)2]BF42O} 3]-) may be achieved in single crystals under very mild conditions, i.e. simply in a stream of dry dinitrogen for ca .2 hrs at room temperature! Color and morphology of the crystals do not change, but the half-width of the reflections increases. The molecular and crystal structures of both the diaqua complex in fig 1 and the monoaqua reaction product (showing shorter Co-Eu distances and a larger Co-Eu-Co angle) are closely related: The space group symmetry is retained, lattice constants are very similar, and the cell volume decreases only slightly during reaction. The reaction is reversible - a crystal of the monoaqua complex takes up water and reverts to the starting complex under ambient conditions! Experiments on oriented single crystals reveal the close relation between the two unit cells, fig 2.
In contrast to our results, most solid state reactions of molecular crystals yield only microcristalline or amorphous reaction products.
