DIHYDRODIAZAPHOSPHININES IN THE SYNTHESIS OF MOLECULES OF BIOLOGICAL INTEREST. A. Gutiérrez-Rodrígueza, R. Santiago-Garcíaa, S. García-Grandaa, J. Barluengab, M. Tomásb, K. Biegerb.(a) Departamento de Química Física y Analítica. Universidad de Oviedo. 33071 Oviedo, Spain. (b)Instituto de Química Organometálica E. Moles
Dihydrodiazaphosphinines 1 have been firstly prepared at the University of Oviedo. We have studied their behavior against electrophiles,1,2 particularly dimethylacetylenedicarboxylate (DMAD), by X-Ray diffraction and NMR Spectroscopy. This is another example of the puzzlesome chemistry unable to progress without structural information.
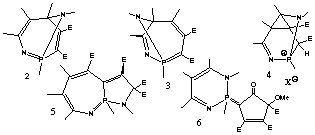
The reaction of 1 with DMAD at -20deg. C yield 2 as mayor product which shows a small C-C-C angle of 96.1deg. at the bridgehead carbon and a short C-C non-bonding distance of 228.05 pm. 2 is unstable and rearranges quantitatively to 3. Both 2 and 3 show similar structures to those of cocaine or atropine. With electrophiles, 2 reacts to policyclic compounds, 4, that co-crystallizes with a molecule of acid.

When 1 reacts with two equivalents of DMAD, a dihydrodiazaazulene system 5 is formed. Finally higher concentrations and even excesses of DMAD lead to the formation of 6.
1. J. Barluenga; M. Tomás; K. Bieger; S. García-Granda; R. Santiago-García; Angew. Chem. Int. Ed. (Accepted for Publication).
2. S. García-Granda; R. Santiago-García; K. Bieger; Acta Cryst. C (Submitted).