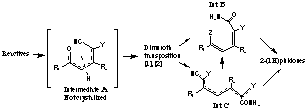
REGIOSELECTIVE SYNTHESIS OF 2-(1H)PIRIDONES. García-Rodríguez, E.(a); García-Granda, S.(a); Yustos Cuesta, P.(b); Alberola Figueroa, A.(b); González Ortega, A.(b). (a) Departamento de Química Física y Analítica. Universidad de Oviedo. Spain, (b) Departamento de Química Orgánica. Universidad de Valladolid.Spain
Piridones are relevant in the pharmacological industry and the studies of their syntetic procedures is of interest, particularly those leading to regioselectivity.
In order to understand the mechanism of the regioselective synthesis of 2-(1H)piridones several products were isolated and characterised by NMR spectroscopy. Since the NMR experiments were not conclusive about the structures, X-Ray diffraction studies were carried out on the following compounds: C14H22N2O3 , C13H19N3O , C17H22N2SO3 (type B), C11H9N4O , C15H9N4O (type C).
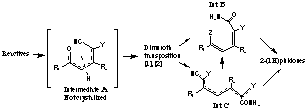
(Z=-Pyrrolidine; Y=-CN,CO2Et,-SO2C6H5;
R1=Me,Et,iPr,Ph,..; R2=H,Me,iPr,Ph,...)
Solid state X-Ray studies provide explanations to most of the NMR questions but are not definitive on the location of H+, present in some compounds. Theoretical calculations and Mass spectrometry will give the final answer.
Wahnen, M. Zeitschrist für Chemie 9, 241-252 (1969)
Brennen, G., Schmidt, H. L. Chem. Ber. 110, 373, (1979)