CRYSTAL STRUCTURES OF ORGANOMETALLIC ALUMINUM AMINO-ALKOXIDES T. Gelbricha) J. Sielera), E. Hechtb), U. Dümichenb)
a) Institut für Anorganische Chemie der Universität, D-04103 Leipzig, Linnéstr. 3,
b) Institut für Anorganische Chemie der Martin-Luther-Universität Halle-Wittenberg,
D-06217 Merseburg, Geusaer Straße, Germany.
Aluminum compounds can be stabilized by donor ligands. This stabilization is especially pronounced when chelating ligands are present [1].
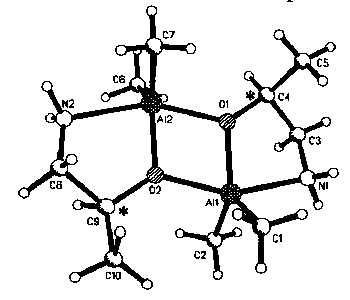
Compounds of general formula Me2AlOR* [OR* = (+);(-)-dimethylamino-2-propoxide 1, (S);(+)-amino-2-propoxide 2, (+);(-)-2-piperidylmethoxide 3] have been characterized by single crystal X-ray structure analysis at 200 K. In the solid state, compounds 1-3 are dimeric. The central four-membered Al2O2 rings are planar and each aluminum is coordinated in a distorted trigonal bipyramidal fashion by two methyl groups (axial), two alkoxide substituends (axial and equatorial) and the N atom of the aminoalkoxide (equatorial). In 1-3 the axial Al-O bond length is significant shorter than the equatorial one. In 1, the center of the A12O2 ring coincides with a crystallographic inversion center (space group P21/c). The absolute of structure of 2 (space group P212121) was found to be (S) (Flack parameter = 0.04). Compound 3 crystallizes with two non-interacting molecules of toluole per formula unit.
[1] Dümichen,U., Thiele, K.-H., Gelbrich T., Sieler, J., J. Organomet. Chem., 495 (1995) 71-75.
Figure 1: Molecular structure of
(S);(+)-amino-2-propoxide 2
