HYDROGEN BONDS IN CRYSTAL STRUCTURES OF TWO COBALT(II) COMPLEXES WITH NITROXIDE. A.B. Burdukov, N.V. Pervukhina. International Tomography Center Sib. Branch of RAS, Novosibirsk, Russia; Institute of Inorganic Chemistry Sib. Branch RAS, Novosibirsk, Russia.



I II
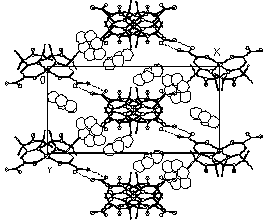
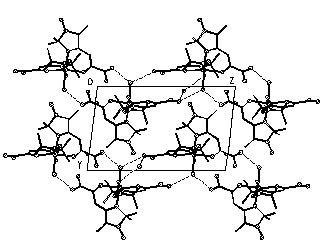
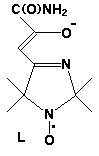
Two new adducts CoL2[[dotaccent]]3C6H6 (I) and CoL2[[dotaccent]]4H2O (II) have been synthesized (I, monoclinic, C2/c, a=22.573(5), b=10.356(2), c=19.040(4) Å, =113.36(3)deg., V=4086(2) Å3, Z=8, Dc=1.21 g*cm-3, 857 Ihkl >2I, R=0.0659; II triclinic, P1(-), a=9.922(1), b=9.977(1), c=15.252(1) Å, =91.17(1), =102.14(1), =113.44(1)deg., V=1345.2(2) Å3, Z=2, Dc=1.43 g*cm-3, 2747 Ihkl >2I, R=0.0428; CAD-4, Mo K for I and II). In I the metal atom has a tetrahedral coordination with Co-O distance 1.951(6), Co-N 1.971(7) Å, OCoN 95.7(6)deg.. The CoL2 molecules are connected into chains by H-bonds O...H-N, the benzene molecules laying between the chains. In II Co(II) has octahedral surrounding including O and N atoms of L and two cis-H2O molecules (Co-O 2.013(3), 2.031(3), Co-N 2.111(3), 2.132(3), Co-Ow 2.199(4), 2.255(3) Å). The coordinated water molecules form H-bonds with non-coordinated H2O, amide- and nitroxide groups of L. The H-bonds seem to be the main factor in the formation of these two structures.