UNEXPECTED CHIRALITY IN THE NOVEL MIXED-METAL CLUSTER COMPOUNDS M2Fe(3-X)(CO)7(5-C5H)2 (M=Mo,W, X=Se, Te). A.V.Virovets, S.N.Konchenko, N.V.Podberezskaya, S.V.Tkachev,E0104 Institute of Inorganic Chemistry SB RAS, Novosibirsk, Russia
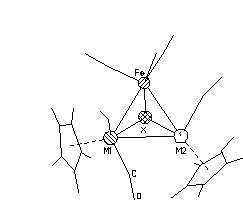
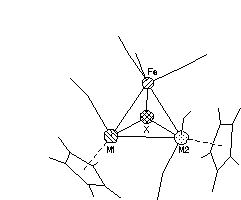
Single crystals of four new mixed-metal clusters were investigated by X-ray structural analysis (X=Se, M2=Mo2, W2 and MoW; X=Te, M2=Mo2). It has been found that in the solid state cluster molecule contains two non-equivalent M-Fe bonds (even if M1=M2) due to the different arrangement of two Cp ligands: the M1 atoms have Cp ligand in cis- position with respect to the M1-Fe bond in contrast to the M2 which have trans- Cp. In molybdenum clusters the difference in the lengths of Mo-Fe bonds is higher when X=Te then in X=Se case (0.133 and 0.025 Å respectively). Such an asymmetrical ligand arrangement makes molecules to be chiral. All crystals are centrosymmetrical and, therefore, they are racemic mixtures of chiral isomers A and B:


A B
The NMR investigations show that fast AB ligand exchange process takes place in the solution. More interesting case is M1=Mo, M2=W, when chirality of ligand arrangement adds to the chirality of tetrahedral cluster core resulting in four different diastereomers present in one single crystal (with crystallographic Mo/W disorder).