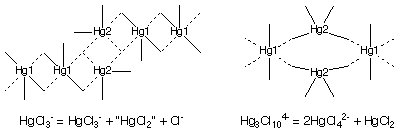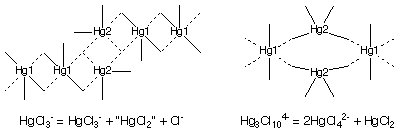
STRUCTURAL DIVERSITY IN CHLOROMERCURY(II) SALTS. Anthony Lindena, Bruce D. Jamesb, John Liesegangc and Nick Gonisb, aInstitute for Organic Chemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; bSchool of Chemistry and cSchool of Physics, La Trobe University, Bundoora, Victoria 3083, Australia.
Chloromercurate complexes are well known for the ability of their anions to exhibit a wide range of geometry, stoichiometry and connectivity. The anions of the salts formed by the addition of 2-, 3- and 4-chloropyridine to HgCl2 in conc. HCl have remarkably different structures. The 2-chloropyridinium salt has the HgCl3- stoichiometry, but the anions consist of infinite chains made up from HgCl3-, distorted HgCl2 and Cl- moieties linked by longer Hg...Cl contacts which range from 2.80 to 3.30 Å. The Cl- ion bridges three Hg centres and is also hydrogen bonded to both of the independent cations. The Hg centres exhibit distorted trigonal bipyramidal and square pyramidal coordination. The 3- and 4-chloropyridinium salts have included an additional Cl- ion to give the Hg3Cl104- stoichiometry. The anions consist of infinite chains made up from HgCl2 and HgCl42- entities interconnected by longer Hg...Cl contacts, so that Hg1 has octahedral coordination, while Hg2 is tetrahedrally coordinated. Although the 3- and 4-chloropyridinium salts have anions constructed in the same fashion, there is still considerable variation between the two structures in terms of the bond lengths and angles within the anionic chains and distortions of the coordination geometry at each Hg centre. Across the two structures, the long Hg...Cl contacts vary from 2.93 to 3.25 Å. A second preparation of the 3-chloropyridinium salt under similar conditions yielded crystals with distorted Hg2Cl62- dimers linked into chains by a single long Hg...Cl contact.