MOLECULAR CHAINS, GRIDS AND CUBICLES: DESIGNING INORGANIC POROUS SOLIDS Pierre Losier, Donald C. MacQuarrie and Michael J. Zaworotko Department of Chemistry, Saint Marys University, Halifax, Nova Scotia, B3H 3C3 Canada
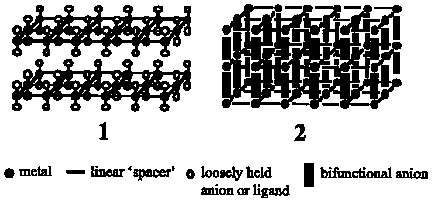
Crystal engineering has lead to the rational design of inorganic polymers containing one- (chains), two- (grids), and three-dimensional (boxes or cubicles) frameworks based upon the established coordination chemistry of the incorporated transition metals (e.g. Co, Ni, Cu, Cd, Zn). Few solids consisting of square planar grids (1) have been reported and the large cavities in these two-dimensional frameworks (>50% by volume) usually lead to interpenetration or enmeshing of one grid into another which results in a reduction or elimination of effective pore size. Recently, we reported the synthesis and structural characterization of an octahedral porous solid (2), [Zn(4,4'-dipyridyl)2]SiF6 *x DMF, which consists of overlapping grids of [Zn(4,4'-dipyridyl)2] linked by the counterion [SiF6]2-. This neutral inorganic polymer eschews interpenetration and has its cavities filled with solvent molecules. We present herein several examples of non-interpenetrated square grid and octahedral inorganic polymers.
