E0476
ANALYSIS OF RIGID BODY AND NON-RIGID BODY MOTIONS IN X-RAY
CRYSTALLOGRAPHY. F. Belaj, Institut für Anorganische Chemie,
Universität Graz, Austria
Due to the high flexibility at the nitrogen atoms the
[P(NPCl3)4]+ cation as a whole cannot be
described as a rigid body not even with allowance for intramolecular torsions
about bonds (J.D. Dunitz, D.N.J. White, Acta Cryst., 1973, A29,
93-94). The analysis of the thermal motion of this cation and of related
species shows that the librations can best be described as almost independent
motions of rigid tetrahedra, coupled by `ball joints'.
An accurate low-temperature structure determination of
[P(NPCl3)4]+ ICl2 was hindered by
phase transition and the room-temperature structure, the cations lying on
 ,
could not be interpreted without some difficulties, too (F. Belaj, Acta
Cryst., 1995, B51, 65-71). Recently the structure determination of
[P(NPCl3)4]+PCl6-
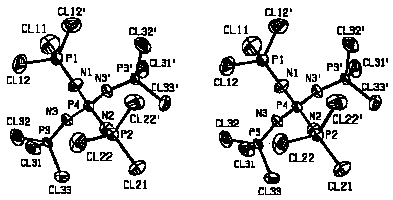
succeeded at 93K: The cations are located on mirror planes, thus three
different NPCl3 groups can be compared (see figure). The P-N bond
lengths and P-N-P bond angles do not show such exceptionalities as in the
ICl2- structure, but similar to the latter the
PN4 and NPCl3 tetrahedra of the cation as well as the
anion behave as rigid bodies with Ru<0.05 {Ru =
[[[Sigma]]w([[Delta]]U)2/[[Sigma]]w(U)2]1/2}
, whereas for the cation as a whole an Ru value of 0.35 is
obtained.
,
could not be interpreted without some difficulties, too (F. Belaj, Acta
Cryst., 1995, B51, 65-71). Recently the structure determination of
[P(NPCl3)4]+PCl6-
succeeded at 93K: The cations are located on mirror planes, thus three
different NPCl3 groups can be compared (see figure). The P-N bond
lengths and P-N-P bond angles do not show such exceptionalities as in the
ICl2- structure, but similar to the latter the
PN4 and NPCl3 tetrahedra of the cation as well as the
anion behave as rigid bodies with Ru<0.05 {Ru =
[[[Sigma]]w([[Delta]]U)2/[[Sigma]]w(U)2]1/2}
, whereas for the cation as a whole an Ru value of 0.35 is
obtained.
 ,
could not be interpreted without some difficulties, too (F. Belaj, Acta
Cryst., 1995, B51, 65-71). Recently the structure determination of
[P(NPCl3)4]+PCl6-
succeeded at 93K: The cations are located on mirror planes, thus three
different NPCl3 groups can be compared (see figure). The P-N bond
lengths and P-N-P bond angles do not show such exceptionalities as in the
ICl2- structure, but similar to the latter the
PN4 and NPCl3 tetrahedra of the cation as well as the
anion behave as rigid bodies with Ru<0.05 {Ru =
[[[Sigma]]w([[Delta]]U)2/[[Sigma]]w(U
,
could not be interpreted without some difficulties, too (F. Belaj, Acta
Cryst., 1995, B51, 65-71). Recently the structure determination of
[P(NPCl3)4]+PCl6-
succeeded at 93K: The cations are located on mirror planes, thus three
different NPCl3 groups can be compared (see figure). The P-N bond
lengths and P-N-P bond angles do not show such exceptionalities as in the
ICl2- structure, but similar to the latter the
PN4 and NPCl3 tetrahedra of the cation as well as the
anion behave as rigid bodies with Ru<0.05 {Ru =
[[[Sigma]]w([[Delta]]U)2/[[Sigma]]w(U