E0716
DIMETHYLSULFOXIDE SOLVATES OF BISMUTH(III) IODIDE. Brian W. Skelton,
Peter C. Junk and Allan H. White, Department of Chemistry, University of
Western Australia, Nedlands, Western Australia 6907, Australia
At room temperature, bismuth(III) iodide crystallises from solutions of
dimethylsulfoxide (dmso) in at least two substantial, well-defined crystalline
solvates, BiI3.2dmso (orange) and BiI3.8/3dmso (red).
BiI3.2dmso is triclinic P
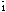 ,
a 12.558(2), b 8.962(2), c 8.342(1)Å, ( 61.85(1),
( 78.27(1), ( 76.89(2)( Z=2, conventional R on |F| being 0.048
for NO 1953 independent `observed' reflections. The complex
is a centrosymmetric binuclear array, one dimer in the unit cell:
[(O-dmso)2I2Bi((-I)2BiI2(O/i>-dmso)2],
bismuth atoms being six-coordinate with the dmso oxygen atoms trans.
Bi-O are 2.37(2),2.41(2), Bi-I(terminal) 2.941(7),2.922(9) and Bi-I(bridging)
3.211(7),3.25(1)Å. Bi-O-S are 123.9(7),125.8(7) and Bi-I-Bi 96.03(7)(.
The badly-twinned crystals of BiI3.8/3dmso are also triclinic P
,
a 12.558(2), b 8.962(2), c 8.342(1)Å, ( 61.85(1),
( 78.27(1), ( 76.89(2)( Z=2, conventional R on |F| being 0.048
for NO 1953 independent `observed' reflections. The complex
is a centrosymmetric binuclear array, one dimer in the unit cell:
[(O-dmso)2I2Bi((-I)2BiI2(O/i>-dmso)2],
bismuth atoms being six-coordinate with the dmso oxygen atoms trans.
Bi-O are 2.37(2),2.41(2), Bi-I(terminal) 2.941(7),2.922(9) and Bi-I(bridging)
3.211(7),3.25(1)Å. Bi-O-S are 123.9(7),125.8(7) and Bi-I-Bi 96.03(7)(.
The badly-twinned crystals of BiI3.8/3dmso are also triclinic P
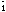 ,
a 16.435(6), b 14.926(2), c 12.396(3)Å, ( 74.89(2), (
73.24(2), ( 79.18(2)(, Z=6, R 0.059 for NO
5858. The complex is
[Bi(O-dmso)8]3+[I3Bi((-I)3BiIsub>3]3-
(i.e. [Bi2I9]3- ) the bismuth environment of
the novel cation being, unusually, dodecahedral rather than square
anti-prismatic, the `inner' sites of the trapezoidal planes having perhaps
slightly longer Bi-O (2.41(2)-2.49(2)Å) than the peripheral
(2.36(2)-2.44(2)Å). In the anion, Bi-I(bridging) are 3.152(2)-3.337(3)
and Bi-I(terminal) 2.903(3)-3.012(2)Å. In the cation, half of the ligand
sulfur atoms are disordered in the usual way; for the more precisely determined
ordered species, Bi-O-S are 118.8(9)-131(1)(.
,
a 16.435(6), b 14.926(2), c 12.396(3)Å, ( 74.89(2), (
73.24(2), ( 79.18(2)(, Z=6, R 0.059 for NO
5858. The complex is
[Bi(O-dmso)8]3+[I3Bi((-I)3BiIsub>3]3-
(i.e. [Bi2I9]3- ) the bismuth environment of
the novel cation being, unusually, dodecahedral rather than square
anti-prismatic, the `inner' sites of the trapezoidal planes having perhaps
slightly longer Bi-O (2.41(2)-2.49(2)Å) than the peripheral
(2.36(2)-2.44(2)Å). In the anion, Bi-I(bridging) are 3.152(2)-3.337(3)
and Bi-I(terminal) 2.903(3)-3.012(2)Å. In the cation, half of the ligand
sulfur atoms are disordered in the usual way; for the more precisely determined
ordered species, Bi-O-S are 118.8(9)-131(1)(.
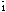 ,
a 12.558(2), b 8.962(2), c 8.342(1)Å, ( 61.85(1),
( 78.27(1), ( 76.89(2)( Z=2, conventional R on |F| being 0.048
for NO 1953 independent `observed' reflections. The complex
is a centrosymmetric binuclear array, one dimer in the unit cell:
[(O-dmso)2I2Bi((-I)2BiI2(O/i>-dmso)2],
bismuth atoms being six-coordinate with the dmso oxygen atoms trans.
Bi-O are 2.37(2),2.41(2), Bi-I(terminal) 2.941(7),2.922(9) and Bi-I(bridging)
3.211(7),3.25(1)Å. Bi-O-S are 123.9(7),125.8(7) and Bi-I-Bi 96.03(7)(.
The badly-twinned crystals of BiI3.8/3dmso are also triclinic P
,
a 12.558(2), b 8.962(2), c 8.342(1)Å, ( 61.85(1),
( 78.27(1), ( 76.89(2)( Z=2, conventional R on |F| being 0.048
for NO 1953 independent `observed' reflections. The complex
is a centrosymmetric binuclear array, one dimer in the unit cell:
[(O-dmso)2I2Bi((-I)2BiI2(O/i>-dmso)2],
bismuth atoms being six-coordinate with the dmso oxygen atoms trans.
Bi-O are 2.37(2),2.41(2), Bi-I(terminal) 2.941(7),2.922(9) and Bi-I(bridging)
3.211(7),3.25(1)Å. Bi-O-S are 123.9(7),125.8(7) and Bi-I-Bi 96.03(7)(.
The badly-twinned crystals of BiI3.8/3dmso are also triclinic P
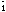 ,
a 16.435(6), b 14.926(2), c 12.396(3)Å, ( 74.89(2), (
73.24(2), ( 79.18(2)(, Z=6, R 0.059 for NO
5858. The complex is
[Bi(O-dmso)8]3+[I3Bi((-I)3BiIsub>3]3-
(i.e. [Bi2I9]3- ) the bismuth environment of
the novel cation being, unusually, dodecahedral rather than square
anti-prismatic, the `inner' sites of the trapezoidal planes having perhaps
slightly longer Bi-O (2.41(2)-2.49(2)Å) than the peripheral
(2.36(2)-2.44(2)Å). In the anion, Bi-I(bridging) are 3.152(2)-3.337(3)
and Bi-I(terminal) 2.903(3)-3.012(2)Å. In the cation, half of the ligand
sulfur atoms are disordered in the usual way; for the more precisely determined
ordered species, Bi-O-S are 118.8(9)-131(1)(.
,
a 16.435(6), b 14.926(2), c 12.396(3)Å, ( 74.89(2), (
73.24(2), ( 79.18(2)(, Z=6, R 0.059 for NO
5858. The complex is
[Bi(O-dmso)8]3+[I3Bi((-I)3BiIsub>3]3-
(i.e. [Bi2I9]3- ) the bismuth environment of
the novel cation being, unusually, dodecahedral rather than square
anti-prismatic, the `inner' sites of the trapezoidal planes having perhaps
slightly longer Bi-O (2.41(2)-2.49(2)Å) than the peripheral
(2.36(2)-2.44(2)Å). In the anion, Bi-I(bridging) are 3.152(2)-3.337(3)
and Bi-I(terminal) 2.903(3)-3.012(2)Å. In the cation, half of the ligand
sulfur atoms are disordered in the usual way; for the more precisely determined
ordered species, Bi-O-S are 118.8(9)-131(1)(.