SELF-ASSEMBLY THROUGH HYDROGEN BONDING. CRYSTAL STRUCTURES OF PSEUDO-SPHERICAL CAPSULES. Leticia M. Toledo, Carlos Valdés, Urs Spitz, Julius Rebek Jr. Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts, 02139 USA
Complementarity of shape, size and chemical surface drives molecular recognition processes and self-complementarity is the singular feature of biological molecules capable of assembly. We have described a series of compounds, whose structures are self-complementary and, thus contain the code for self-assembly.1 These new entities are composed of identical monomeric units held together by intermolecular interactions, in the fashion of palindromic nucleic acid and viral capsids.
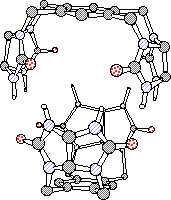
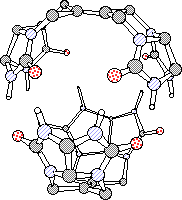
The monomers, symmetrical bisglycoluril molecules, are interlocked into pseudo-spherical dimers by hydrogen bonds in the same way that stitches along the seam hold together a tennis ball. Upon self-association in solution, internal cavities are generated and guest of appropriate sizes can be encapsulated within them.


The crystal structures of two of these capsules have been determined. These
crystallize in
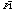 and
and
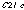 space groups, respectively. Both capsules share similar packing arrangements
in the solid and both contain disordered guests in their internal cavities.
space groups, respectively. Both capsules share similar packing arrangements
in the solid and both contain disordered guests in their internal cavities.
1. C. Valdés, U. P. Spitz, L. M. Toledo, S. W. Kubik, J. Rebek Jr., J. Am. Chem. Soc. 1995, 117, 12733, and references within.