E0882
OXYGEN DEFECT STRUCTURE IN La2MO4+d (M = Cu, Ni,
Co). W. Paulus1,2, A. Cousson1, G. Heger2,
A. Revcolevschi3, G. Dhalenne3, S. Hosoya4, V.
Kvardakov5. 1CE-Saclay, Lab. Léon Brillouin,
F-91191 Gif-sur-Yvette; 2RWTH Aachen, Inst. of Crystallography,
Jägerstr. 17-19 D-52056 Aachen, Germany; 3Lab. of Solid State
Chemistry, Univ. Paris-Sud, F- 91405 Orsay, France; 4Univ. of
Kofu, Institute of Inorganic Synthesis, Kofu 400, Japan;
5Kurchatov Institute Russian Science Centre, 123182 Moskau,
Russia.
La2MO4 (M = Cu, Ni, Co) are isostructural
(K2NiF4-type structure) and can incorporate oxygen on
interstitial sites forming La2MO4+d. The amount of the
extra oxygen is limited to d = 0.08, 0.16 and 0.25 for the Cu, Ni and Co
homologous compounds respectively. With the formation of the oxygen-rich
phases, strong changes in the physical properties are observed, e.g. the
semiconductor La2CuO4.00 becomes metallic and
superconducting with a Tc max. of 44K when oxygenated to d = 0.08.
For the Ni and Co compounds no superconducting behaviour was observed for any
oxygen stoichiometry. In all cases, however, a continuous vanishing of all
a
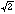 * a
* a
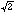 *
c superstructure reflections goes along with the uptake of oxygen. These
superstructure reflections are related to the tilting of the MO6
octahedra observed for stoichiometric La2MO4.00
compounds in the orthorhombic phase at ambient temperature. The purpose of our
studies is to understand the structural disorder induced by the uptake of extra
oxygen in order to estimate the influence on phase transitions and electronical
properties. We report here on our temperature-dependent neutron diffraction
studies carried out on single crystals of La2CoO4+d and
discuss the results in relation with those obtained for
La2NiO4+d. and superconducting
La2CuO4+d.
*
c superstructure reflections goes along with the uptake of oxygen. These
superstructure reflections are related to the tilting of the MO6
octahedra observed for stoichiometric La2MO4.00
compounds in the orthorhombic phase at ambient temperature. The purpose of our
studies is to understand the structural disorder induced by the uptake of extra
oxygen in order to estimate the influence on phase transitions and electronical
properties. We report here on our temperature-dependent neutron diffraction
studies carried out on single crystals of La2CoO4+d and
discuss the results in relation with those obtained for
La2NiO4+d. and superconducting
La2CuO4+d.
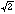 * a
* a
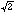 *
c superstructure reflections goes along with the uptake of oxygen. These
superstructure reflections are related to the tilting of the MO6
octahedra observed for stoichiometric La2MO4.00
compounds in the orthorhombic phase at ambient temperature. The purpose of our
studies is to understand the structural disorder induced by the uptake of extra
oxygen in order to estimate the influence on phase transitions and electronical
properties. We report here on our temperature-dependent neutron diffraction
studies carried out on single crystals of La2CoO4+d and
discuss the results in relation with those obtained for
La2NiO4+d. and superconducting
La2CuO4+d.
*
c superstructure reflections goes along with the uptake of oxygen. These
superstructure reflections are related to the tilting of the MO6
octahedra observed for stoichiometric La2MO4.00
compounds in the orthorhombic phase at ambient temperature. The purpose of our
studies is to understand the structural disorder induced by the uptake of extra
oxygen in order to estimate the influence on phase transitions and electronical
properties. We report here on our temperature-dependent neutron diffraction
studies carried out on single crystals of La2CoO4+d and
discuss the results in relation with those obtained for
La2NiO4+d. and superconducting
La2CuO4+d.