BONDING IN METAL-ALKYNE COMPLEXES: CRYSTALLOGRAPHIC STUDIES OF REDOX PAIRS. Michael J. Quayle and A. Guy Orpen. School of Chemistry, University of Bristol, Bristol BS8 1TS, U.K.
Structural changes occuring as a result of one electron oxidation may be used as a probe of electronic structure. In this study we report a study of metal-alkyne bonding based on redox pair structures determined by low-temperature X-ray crystallography.
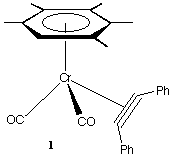
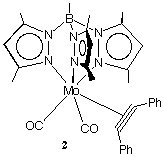
The complexes studied include [Cr(CO)2([[eta]]2-C2Ph2) ([[eta]]6-C6Me5H)] (1) and [Mo(CO)2([[eta]]2-C2Ph2)Tp'] (2) (see below).1 In each case both the neutral and cationic complexes were studied. Formally these are sixteen (2+), seventeen (1+, 2), and eighteen (1) electron alkyne complexes. In practice the alkyne may act in the limit as either a two-electron or a four-electron donor. In the latter case there is a [[pi]] -symmetry interaction between an empty d-orbital on the metal and a filled [[pi]]^ orbital on the alkyne.2 The structural changes observed are significant and together with Extended Hückel MO and ESR spectroscopic studies provide a detailed insight into the nature of the metal-alkyne bonding in these and related systems


References.
1. N.G. Connelly et al J. Chem. Soc. Chem. Comm., 1992, 1293 and unpublished work
2. J.L. Templeton et al J.Am Chem. Soc., 1981, 103, 7713.