CRYSTAL CHEMISTRY OF LAYERED VANADIUM OXIDE CATHODE MATERIALS. Peter Y. Zavalij, M. Stanley Whittingham. State University of New York at Binghamton, Binghamton, NY 13902-6016, USA
Structural analysis of V2O5 layers as well as VO3 and V3O8 polyanions has been done based on known structures with square pyramidal coordination of vanadium and stoichiometry V2O5, MxV2O5, MXVO3, MXV3O8, MXV6O15, etc. including our new compounds (LiV2O4.H2O, TMAV4O10, TMAV2O5 and DTAV3O8, where TMA = tetramethyl ammonium, DTA = dodecyl trimethyl ammonium) with novel topography of layers.
The unique layered structures of vanadium oxides and their derivatives are of particular interest because of their capacity to intercalate lithium and other cations between their layers that makes them promising candidates for cathode materials in secondary lithium batteries.
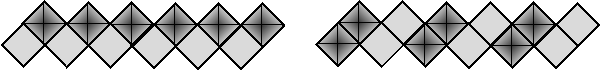
The most common V-polyhedron is a square pyramid VO5 with double bonded oxygen in its vertex. Those polyhedra when sharing edges form double chain with stoichiometry VO3 which exist by itself

in KVO3.H2O and Co(VO3)2.4H2O. The topography of the double chains can be simply presented by symbolic formula using two symbols (U - up and D - down) which show the orientation of the square pyramid. The symmetry of the formula by using simple rules leads to 10 possible symmetry groups of the double chains. In most cases those double chains form layers by sharing single bonded oxygen atoms of basis. Using different ways of joining chains to the layers the possible symmetry, topography, and dimensions of V2O5 layers has been developed. Those conclusions are used to predict the structure of the layers in the novel compounds. The structure of V2O5 layers is also discussed from the point of view of their capacity to easily accept additional charges that yields rich intercalation opportunities in contrary to frameworks constructed with VO4 tetrahedra.