X-RAY CRYSTALLOGRAPHIC, NMR AND KINETIC CORRELATION OF SELECTED Pt(II) COMPLEXES. S. Otto; A. Roodt, Department of Chemistry; University of the Orange Free State; Bloemfontein; 9300; South Africa; e-mail: CHJJ@UOVSVM1.uovs.ac.za
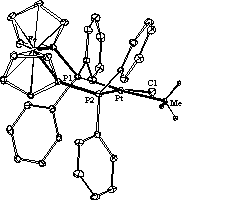
A variety of related Pt(II) compounds were synthesised and characterised with the aid of various physical techniques, such as X-ray crystallography and multi nuclear NMR spectroscopy. In general the compounds contained one labile group, Cl- in this case; one strong ( donating ligand like H-, CH3- or C6H5- and two tertiary phosphine1 or arsine2 ligands which could be either cis or trans to one another. Some lesser known mono- and bidentate ferrocene containing phosphine ligands were among those used in the study. The aim of the investigation was to manipulate the reactivity of the Pt(II) centre and to bring the observed kinetic results in correlation with the obtained physical parameters like bond distances, chemical shifts and coupling constants of the various ligands under investigation. One example of a compound investigated in this study is:

1Otto, S., Roodt, A., Leipoldt, J.G. (1995). S. Afr. J. Chem. 48, 3/4, 92
2Otto, S., Roodt, A. (1995). Acta Cryst. C51, 1105