CHARGE DENSITY OF KDP(KH2PO4) BY THE MAXIMUM ENTROPY METHOD. Shigefumi Yamamura, Satoshi Kasahara, Masaki Takata and Makoto Sakata, Department of Applied Physics, School of Engineering, Nagoya University, Nagoya, 464-01, Japan
In order to investigate the nature of hydrogen bond in KDP(KH2PO4), the charge density distribution of KDP is obtained at room temperature by the Maximum Entropy Method (MEM) from X-ray powder diffraction data. It is well known that KDP is the prototype of hydrogen-bonded ferroelectrics in which the hydrogen atoms are considered to play an important role in the phase transition and that the phase transition temperature is increased about 100K when hydrogen atoms in KDP are substituted by deuterium atoms. In order to understand such a large isotope effect, there have been performed many studies on the hydrogen bond both theoretically and experimentally. In this study, the nature of hydrogen bond of KDP is described through the charge density distribution. Considering much less scattering power of hydrogen for X-ray than the rest of atoms, it is not an easy task to detect the precise charge density distribution of hydrogen bond by MEM. So far, only one example is known which is ice(Ih) (Sakata, Takata, Oshizumi, Goto and Hondoh (Physics and Chemistry of Ice, 1992, 62-68)). In this case, only oxygen atoms are involved other than hydrogen atoms.
The data set used in the analyses are collected by powder X-ray diffraction
experiments at Photon Factory, Tsukuba. The wave length of incident X-ray
photons is 1.3Å and all the data up to 125 degree in 2-theta which
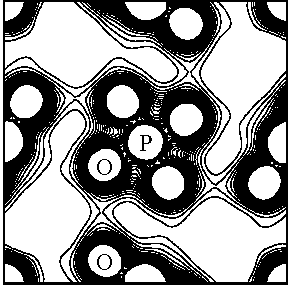
correspond 0.74Å resolution. One of the results is shown in Fig. 1, in
which not only fairly strong PO4 covalent bond but also hydrogen
bond between O - O atoms are recognized. It seems that the nature of hydrogen
bond is weak covalent bond rather than ionic between fairly long distant two
oxygen atoms. It is also found that the charge density at the mid point of O -
O in KDP is higher than that in ice(Ih), which must be the
reflection of strength of hydrogen bond in these materials. Indeed, O - O bond
length in KDP is shorter than that in ice(Ih).
