ELECTRON DENSlTY STUDIES ON METAL TRISDlTHIOCARBAMATE AND METAL BIS-DITHIOCARBAMATE COMPLEXES. By Li-Ya Tan, Chi-Rung Lee, Chih-Chieh Wang, & Yu Wang, Department of Chemistry, National Taiwan University, Taipei Taiwan, R.O.C.
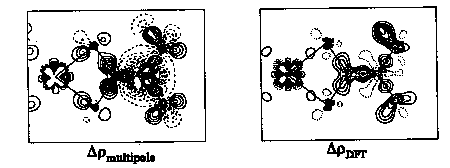
Electron density distributions on three metal dithiocarbamate complexes, M(S2CNR2)3, M=Fe(III), Co(III), and Ni(S2CNR2)2, are studied by accurate single X-ray diffraction data measured at 140K using MoK[[alpha]] radiation. The metal ions of the former two compounds are 6-coordinated with a geometry between trigonal prism and octahedron. The twist angles of MS6 polyhedron viewed from C3-axis are ca 33deg. for Fe(S2CN(C4H8))3, and ca .46deg. for Co(S2CNMe2)3 (60deg. for ideal octahedron and 0deg. for ideal trigonal prism). The Nickel(II) in the complex Ni(S2CNEt2)2 is in a 4-coordinated planar geometry with Ni at 1 position. Detail electron density distributions will be presented for bonding characterization.The interesting d-orbital populations on these high spin d5 Fe(III), low spin d6 Co(III), and d8 Ni(II) will be compared. A comparison between experiment and molecular orbital calculation is also going to be discussed. The agreement between experimental results and MO calculations is good in the case of Co(III) complex(see figures below), but not so good in the other two cases.
