THE REACTIVITY OF SOLID BENZOPHENONES TOWARDS SOLID-STATE REDUCTION: STRUCTURAL AND THERMODYNAMIC ASPECTS. Dirk Abeln, Stefan Ebbinghaus, Jürgen Kopf and Matthias Epple, Institute of Inorganic and Applied Chemistry, University of Hamburg, MartinLuther-King-Platz 6, D-20146 Hamburg, Germany
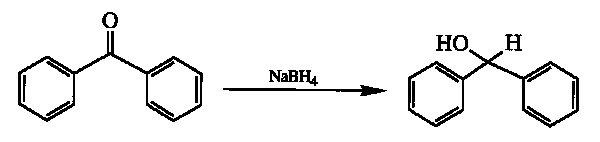
Benzophenone can be reduced to benzhydrol by simply mixing the solid compound with solid sodium borohydride (NaBH4) and leaving the mixture at room temperature for a few days [1,2]:
This interesting reaction could be of interest as a "reaction without solvent", that is highly desirable for ecological, economic and toxicological reasons.
We studied the kinetics and the feasibility of this reduction reaction for a number of substituted benzophenones. The crystal structure should play an important role for this kind of solid-state reaction, therefore the structures of a number of benzophenones and benzhydroles were determined by single-crystal X-ray diffractometry. Additionally, experiments with thermal analysis (DSC), scanning electron microscopy and NMR were performed. In some cases this reaction, which was originally postulated as pure solid-state reaction [1], occurs via intermediate liquid phases. These could be identified as eutectic mixtures of benzophenone and benzhydrol.
[1] F.Toda, K. Kiyoshige, M. Yagi, Angew. Chem. Int. Ed. Engl. 28 (1989) 320-321.
[2] M.Epple, S. Ebbinghaus, A. Reller, U. Gloistein, H.K. Cammenga, Thermochim. Acta 269/270 (1995) 433-441.
