KETOACID REDUCTOISOMERASE: FIRST STRUCTURE OF A POTENTIAL HERBICIDE TARGET. Valerie Biou, Eva Pebay-Peyroula, Claudine Cohen-Addad, Institut de Biologie Structurale, Grenoble, France; Renaud Dumas, Dominique Job & Roland Douce, Laboratoire Mixte CNRS-Rhône-Poulenc, Lyon, France.
Ketoacid Reductoisomerase (KARI) takes part in the synthesis of Valine and Isoleucine in plants and micro organisms. KARI is a dimer of 60 kDa per monomer, and it has several exciting characteristics: it specifically recognizes two different substrates with a micro molar affinity, and the reaction requires the presence of NADPH and two Mg ions. This biosynthesis pathway is absent from animals and therefore this enzyme is a good potential target for a rational search for herbicides. Two molecules have been found to inhibit KARI and have herbicidal effects at high doses. They both are analogues of the reaction intermediate and are competitive of the substrate. We present here the structure at 1.65Å resolution of the spinach enzyme overexpressed in E. coli, crystallised in the presence of one inhibitor, IpOHA, and of NADPH and Mg ions.
The structure was solved using the Multiple Isomorphous Replacement method and non crystallographic symmetry averaging. The electron density map clearly shows the presence of two hexa-liganted magnesium ions in the active site. It also reveals interactions between the inhibitor, the protein and the NADPH. Details of those interactions will be presented, with some insight as to how the reaction is performed by KARI.
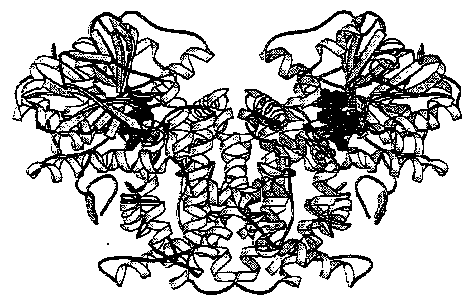
Figure 1: KARI dimer. The ligands are represented with spherical atoms: NADPH in dark grey, Mg++ in black and IpoHa in lighter grey. Program Molscript (P. Kraulis, J. Appl. Cryst. (1991), 24, 946-950) was used to generate the figure.
