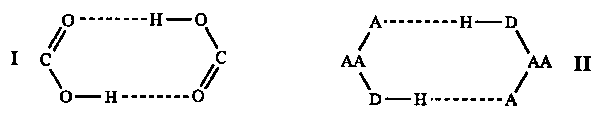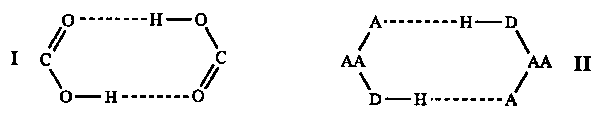
HYDROGEN-BOND PATTERN FUNCTIONALITY AND GRAPH SETS. J. Bernstein, Department of Chemistry, Ben-Gurion University of the Negev, Beer Sheva, Israel 84965, and R. E. Davis, Department of Chemistry, University of Texas, Austin,TX 78712, U.S.A.
One of the most promising approaches for defining and understanding hydrogen-bond patterns in crystals and multimolecular arrays was developed by the late M.C. Etter (1990), who applied a shorthand graph theory to recognize, summarize and then to utilize patterns of hydrogen bonding. There has been an increasing use and recognition of the power of the method, and the very formalism of the procedure has led to new insights about characterizing and classifying arrays of molecules (Bernstein et al, 1995). This efficient shorthand notation for describing hydrogen-bonded networks makes the patterns easy to describe, compare and recall, and a new language describing the structures of micro- and macromolecular ensembles is emerging.
In the course of developing and refining this new language we have come to recognize the existence of hydrogen-bond pattern functionality that is based on properties of molecular recognition dictated by the characteristics of hydrogen bonding but that cuts across traditional lines of chemical functionality. For instance, the ring pattern commonly associated with by carboxylic acids, I, has been found for eighty different combinations (i.e. chemical functional groups, II( AA = any atom, D= hydrogen bond donor, A = hydrogen bond acceptor), thus demonstrating how this hydrogen-bond pattern functionality crosses the boundaries of traditional chemical group functionality.
We will review the basics tenets of the use of graph sets to characterize hydrogen-bond networks, and will provide examples demonstrating the recognition and potential use of hvdrogen-bond pattern functionality.
ETTER, M.C. (1990). Acc. Chem. Res. 23, 120.
BERNSTEIN, J., DAVIS, R.E., SHIMONI, L., CHANG, N.-L. (1995).
Angew. Chem. Int. Ed. Engl. 34, 1555-1573.