THE CRYSTAL CHEMISTRY OF ALKALOIDS, NATURE'S EXOTIC MOLECULES, Robert O. Gould, Chemistry Department, The University of Edinburgh, EDINBURGH EH9 3JJ. U.K
Alkaloids are natural products which have evolved in plants, normally for a defensive purpose. They are usually highly asymmetrical, and rather bizarre in appearance. In addition to at least one basic nitrogen centre, they contain a specific collection of functional groups spaced out to recognise and interact with particular receptors, often the proteins of the taste buds of would-be predators. Their high degree of selectivity extends to their tendency to co-crystallise with a range of other materials, usually the anions of acids. This property has been used for over 100 years in the resolution of enantiomeric substances. Many of the classical resolution crystals have yet to have crystal structures reported, and only a few attempts have been made to interpret structural differences between a pair of diastereomeric structures.
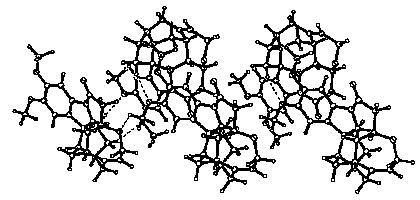
The talk will mainly concentrate on attempts to correlate the types of structure found in cocrystals of brucine, an alkaloid closely related to strychnine, and like it extracted from the seeds of plants of the Strychnos genus. This molecule is sufficiently large, that many of its crystals involve a significant amount of "self-recognition", leading to the formation of layers with a relatively few surface topologies. The generally hydrophobic nature of the much of the molecule enables it to form a core, the surface of which is available for hydrophilic interactions. A ribbon structure, characteristic of several crystals containing brucine is shown in the figure.
