SOLID-STATE PHOTOISOMERIZATION OF 4-BUTEN-1-YL COBALOXIME COMPLEXES. Taro Yamada and Yuji Ohashi Department of Chemistry, Tokyo Institute of Technology, Meguro-ku, Tokyo 152
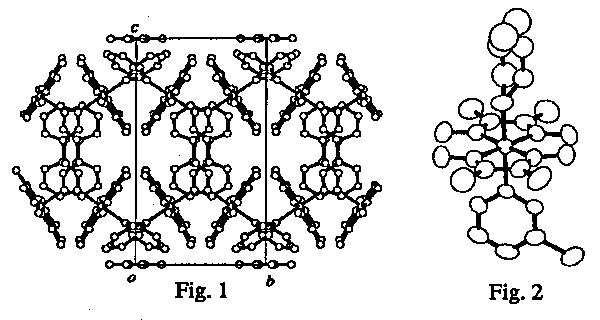
We have found that trans-2-buten-1-yl cobaloxime complexes isomerize to cis isomer without degradation of the single crystal form on exposure to visible light. Recently we prepared several 4-buten-1-yl cobaloxime complexes with different axial base ligands. 1H-NMR measurement showed the complex isomerized to the 2-buten- 1-yl cobaloxime complex when it was irradiated with a Xe-lamp for a day. This means allylic migration of the methylenic hydrogen atom occurred in the solid-state. In order to examine the reason why the migration can occur, the crystal structures of the 4-buten-1-yl complexes with triphenylphosphine, 3-chloropyridine, aniline, and water as axial base ligands were analyzed by X-rays. The space group of the 3-chloropyridine complex crystal is P2/a and two 4-butenyl groups related by a 2-fold axis face each other in the crystal structure (Fig. 1). The molecular structure (Fig. 2) showed disordered 4-butenyl group. Such disordered structures were also observed in the other crystals. The structural change from 4-buten-1-yl to 2-buten-1-yl may easily occur because of the disordered structure with large void space.
