SOLID STATE PHOTOISOMERIZATION OF COBALOXIME COMPLEX IN DIFFERENT CLATHRATE CRYSTALS.Daisuke Hashizume and Yuji Ohashi, Department of Chemistry, Tokyo Institute of Technology, Japan
The reactivity in the solid state can be controlled by the environment of the reactive group. The 2-cyanoethyl group bonded to the cobalt atom in some cobaloxime complexes is isomerized to the 1-cyanoethyl group on exposure to visible light in the solid state. For the solid state reaction, the void space, which is called cavity, around the reactive group is an important factor for the rate and the selectivity of the reaction as well as the conformation of the reactive group. As the cavity is made by the surrounding atoms of the reactive group, the reaction can be controlled by the crystal environment.
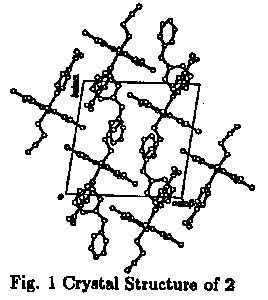
Recently, we have been found that the (2-cyanoethyl)(isonicotinic acid) cobaloxime complex forms clathrate complexes, (1, 2), with dicyclohexylamine and diphenylamine when the cobaloxime complex crystallizes from the methanol solutions containing respective amines.
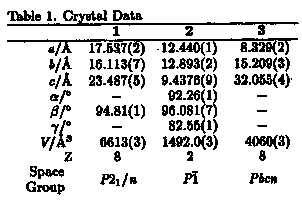
The crystal structure analyses for 1, 2 and the crystal of the guest molecules, 3, were performed. The conformation and the cavity of the reactive group are different among the crystals. The reaction rates are clearly found to be controlled by making the clathrate crystals.

