S0758
CRYSTAL STRUCTURE OF A NEW COORDINATION POLYMER,
{[Ni2Cl2(L)(CH3OH)2(H2O)2]Cl2}
n , FROM X-RAY POWDER DATA. A. Neels, B. Mathez Neels and H.
Stoeckli-Evans, Département de Chimie-Physique II, Université de
Neuchâtel, Switzerland. A. Clearfield and D. M. Poojary, Department of
Chemistry, Texas A & M University, USA.
Coordination metal complexes with polymeric structures are of special interest
in the development of new materials. Molecular ferromagnets and non-linear
optical materials are the most important applications of such coordination
polymers.
2,5-Bis(2-pyridyl)pyrazine (L) has been used as molecular bridge between metal
centers. Some binuclear complexes and a two-dimensional polymer with 3d
transition metals are characterized by their single-crystal X-ray structure
[1].
[[Ni2Cl2(L)(CH3OH)2(H2O)2]Cl2}
n (1), a new one-dimensional coordination polymer, was
obtained in micro-crystalline form. The structure was determined ab initio
from X-ray powder diffraction data. The compound belong to the triclinic
space group P1 with a = 8.7014(4)Å, b = 10.1465(5)Å, c =
8.0303(3)Å, [[alpha]] = 116.095(2)deg.,
[[beta]] = 112.713(3)deg., [[gamma]]= 64.056(3)deg.. The final agreement
factors are Rwp = 0.124, Rp = 0.095, and RF =
0.045
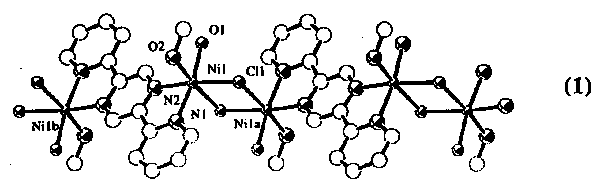
The octahedral coordination of the nickel atom is achieved by two nitrogens of
the ligand 2,5-bis(2-pyridyl)pyrazine, two chlorines and two oxygens of the
coordinated solvent molecules. The bridging nature of chloride ions and
bis-bidentate ligands (L) leads to a one-dimensional polymer.
[1] Neels,A., Stoeckli-Evans,H.; Escuer,A.; Vicente,R.; Inorg. Chem. 34(1995)
1964.