Next: Alcohol dehydrogenase Up: 8. Enzymes and Other Proteins Previous: Hemoglobin
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: Alcohol dehydrogenase
Up: 8. Enzymes and Other Proteins
Previous: Hemoglobin
This enzyme catalyses the hydrolysis of peptide bonds of proteins in the small intestine. It is selective for peptide bonds with aromatic or large hydrophobic side chains (Tyr, Trp, Phe, Met) on the carboxyl side of this bond. Chymotrypsin also catalyses the hydrolysis of ester bonds. X-ray studies have revealed a `charge relay system' of Asp-102, His-57 and Ser-195. This grouping has been found in a whole group of enzymes called the `serine proteases'. Neutron diffraction studies on trypsin show that His-57 acts as a base in the catalytic process. The hydrolysis of peptide bonds occurs by general base-catalysed nucleophilic attack on the carbonyl carbon of the substrate by the hydroxyl oxygen of Ser-195. At the same time the hydroxyl proton of serine is transferred to the imidazole of His-57, the chemical base in the hydrolysis reaction.
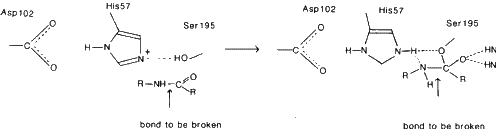
The hydroxyl group of Ser-195 attacks the carbonyl carbon atom of the peptide bond to give a tetrahedral intermediate. His-57 donates a proton to the nitrogen atom of the peptide bond, leading to cleavage and acylation of the enzyme. Deacylation then occurs with water taking the place of the amine group of the substrate.
Blow, D. M., Birktoft, J. J. and Hartley, B. S., Nature 221 (1969) 337; Blow, D. M., in P. B. Boyer (ed.), The Enzymes , Vol. III, 3rd edition, p. 185, New York, London and San Francisco. Academic Press (1971); Kossiakoff, A. A. and Spencer, S. A., Nature 288 (1980) 414.
Copyright © 1984, 1997 International Union of Crystallography
IUCr Webmaster