Next: 8. Quaternary and Other Systems Up: The Study of Metals and Alloys Previous: 6. Phase Diagrams of Binary Alloys
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: 8. Quaternary and Other Systems
Up: The Study of Metals and Alloys
Previous: 6. Phase Diagrams of Binary Alloys
For ternary alloys, two parameters are needed to define each composition and composition diagrams are therefore two-dimensional; no extra dimension is available for representing temperature. We therefore have to represent ternary results by a succession of diagrams, one for each temperature.
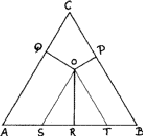
Compositions can be represented in an equilateral triangle; each corner represents an element, and each side a binary system; ternary compositions are represented by points within the triangle, the relative proportions of the elements being given by the lengths of the perpendiculars from the given point to the side of the triangle opposite the appropriate element (Fig. 8). In this figure the point O represents an alloy of 20% A, 30% B and 50% C.
This device is possible because the sum of such perpendiculars is independent of
the position of the point, such as O (Fig. 8). We can see this by drawing
lines - OS and OT - from O parallel to the sides. The length of AS is
clearly (2/![]() )OQ and of BT is (2/
)OQ and of BT is (2/![]() )OP. The triangle
OST is equilateral and therefore TS is equal to (2/
)OP. The triangle
OST is equilateral and therefore TS is equal to (2/![]() )OR. The
total length OP + OQ + OR is thus equal to
)OR. The
total length OP + OQ + OR is thus equal to ![]() ) = AB,
which is a constant.
) = AB,
which is a constant.
 |
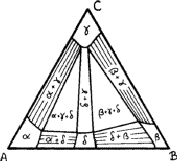
In ternary alloys, one, two or three phases can be in equilibrium at a general
temperature. (At certain specific temperatures four can be in equilibrium.)
Figure 9 shows three solid solutions - ![]() and
and ![]() - based upon
elements A, B and C respectively and another solid solution,
- based upon
elements A, B and C respectively and another solid solution, ![]() , based
upon a binary compound of A and B; it also shows four two-phase regions and
two three-phase regions.
, based
upon a binary compound of A and B; it also shows four two-phase regions and
two three-phase regions.
Within the two-phase regions only specific compositions can be in equilibrium; the lines joining these compositions are known as tie lines . The lines can be found by lattice-parameter methods similar to those used for binary alloys, but now it is necessary to find the variation of lattice parameter along the sides of the single-phase regions; measurement of the lattice parameters in a two-phase alloy should then give the compositions of phases in equilibrium.
There is no reason why tie-lines should behave as regularly as those shown in
the region ![]() in Fig. 9; they can be irregular. There are
thermodynamic reasons for supposing that this type of behaviour is more general.
in Fig. 9; they can be irregular. There are
thermodynamic reasons for supposing that this type of behaviour is more general.
The boundaries between two-phase and three-phase regions must be straight lines, since they are tie lines; if they were not straight, points within the bows of the curves could not represent phases in equilibrium. Thus three-phase regions must be triangular, the corners representing compositions that are in equilibrium. The lattice parameters of the phases within three-phase regions must therefore be constant; only the proportions of the phases differ from point to point. This provides one way of checking that an alloy is three-phase, even if one of the phases is vanishingly small.
Diagrams are usually much more complicated than that shown in Fig. 9. Phases can occur that are not present in any of the binary systems and so will be represented by regions that do not reach the sides of the triangle. Thus, although it is necessary to begin an investigation of a ternary system by exploring the three binary systems, one must always be prepared to find new powder patterns arising.
 |
Copyright © 1984, 1998 International Union of Crystallography
IUCr Webmaster