 |


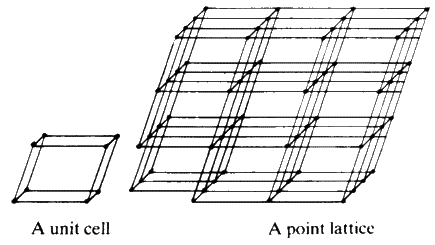
Each of the unit cells (Fig. 7) is identical to all others and the symmetry and shape of the whole crystal depends on the symmetry of that cell.
In general, materials can be divided into various classes according to the bonding holding the solid together. Metals constitute one well-known class. Typical organic compounds, such as naphthalene, crystallize into what are called molecular or van der Waals solids. These are characterized by very low melting points because the melting point is a function of the weak attractions between the organic molecules. Very hard materials like quartz and diamond are typical of crystals which are held together by a 3-dimensional network of very strong covalent bonds. Sodium chloride is a typical example of an ionic solid.
The ideas of the unit-cell, the lattice and the symmetry of crystals are abstractions which can be discussed without reference to specific crystals. But in order to come to a real understanding of the subject it is important to gain first hand experience of how the abstractions operate in real materials by a careful study of crystal models.
What is said about the symmetry of the unit cells or about the symmetry of the faces of the crystals, or any other comment that is made about the lattices or the Miller indices, applies equally well to all possible classes of crystalline material. It does not matter which we are dealing with.
First and foremost in importance is the idea of symmetry. Does the molecule that we are going to deal with have certain symmetry properties? Does the crystal that we are going to look at have certain symmetry properties? Probably the best place to start with symmetry is your left hand; it has symmetry 1: it cannot be converted into itself by any rotation or movement. Similarly, your right hand has symmetry 1: it cannot be transformed into itself by any motion. However, if you place the two hands together so the palms are up and the little fingers are touching edge to edge, then down the line where the two hands join there is a mirror plane. In other words, if the left hand is removed and a mirror is placed next to the right hand, the reflection in the mirror is to all intents and purposes exactly the same as the left hand so these two hands stuck together have the symmetry m, meaning mirror symmetry. If you place the two hands palm to palm, the same thing applies (see Fig. 8). There is a mirror plane of symmetry where the two palms touch; so if you remove the right hand and place a mirror there and look into it, the left hand appears on the right hand side behind the mirror and it looks just the way the right hand did. If you put the two hands back together, right where the two palms touch is a mirror plane of symmetry.
Now make the two thumbs touch at their tips and look at the backs of the hands. Again there is a mirror plane between the hands. Now rotate one hand about the point of contact of the thumbs so that the fingers of each hand point in opposite directions. The back of one hand and the palm of the other are now visible.
The two hands are now related by what is called a centre of inversion at the point of contact of the thumb. The hands can be separated and, if maintained in the same relative orientation there will still be a centre of inversion between them (Fig. 9).
Mathematically speaking, if the coordinates of any point on one hand, relative to the centre of inversion, are x, y and z, then the coordinates of the corresponding point on the other hand will be -x, -y and -z.
To a first approximation, a person has a mirror plane of symmetry
splitting him in half right down the middle of the face and the body. If
two people link arms with bent elbows so that one faces forward and the
other faces back then that pair (as long as they are both male or both
female) has approximately a two-fold axis running parallel to the line of
their bodies, perpendicular to the floor, through the point where the two
elbows meet. If they turn around 180![]() , they are (for all intents and
purposes) the same as they were when they started. They would represent
a molecule which has a two-fold axis of symmetry.
, they are (for all intents and
purposes) the same as they were when they started. They would represent
a molecule which has a two-fold axis of symmetry.
Probably the best way to get a feeling for symmetry elements in molecules and in crystals, is to draw a few yourself, look at a fair number of illustrations in the reference text books and, as mentioned earlier, study crystal models.
Copyright © 1981, 1997 International Union of Crystallography
IUCr Webmaster