Advances in membrane protein crystallography
 Membrane protein structural biology has made tremendous advances over the last decade [Weyand & Tate (2015). Acta Cryst. D71, 1226-1227; doi:10.1107/S1399004715008317], as indicated by the exponential growth in the number of structures that have been published. These advances are a result of many factors including improvements in membrane protein overexpression, stabilization of proteins using antibodies or thermostabilizing mutations, and the enhancement of crystallization technologies such as crystallization in lipidic cubic phase (LCP).
Membrane protein structural biology has made tremendous advances over the last decade [Weyand & Tate (2015). Acta Cryst. D71, 1226-1227; doi:10.1107/S1399004715008317], as indicated by the exponential growth in the number of structures that have been published. These advances are a result of many factors including improvements in membrane protein overexpression, stabilization of proteins using antibodies or thermostabilizing mutations, and the enhancement of crystallization technologies such as crystallization in lipidic cubic phase (LCP).
However, there are still many challenges associated with membrane protein crystallization, data collection and structure determination. Major problems often arise because membrane proteins frequently form tiny crystals, which either cannot be improved in size or can be improved in size but, as a consequence, lose diffraction quality. In addition, crystal handling, such as mounting the crystals and soaking in cryoprotectants, is often the reason for the loss of diffraction quality through mechanical shear-induced microlesions. This is particularly true for membrane protein crystals, which are often very fragile because of their high solvent content and being very thin in one dimension. Two independent groups, Axford et al. and Huang et al., have published methods that make a major contribution to addressing these problems, which will facilitate high-resolution data collection from fragile crystals.
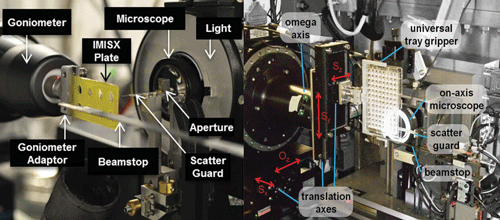
In the methodology demonstrated by Axford et al. [Acta Cryst. (2015). D71, 1228-1237; doi:10.1107/S139900471500423X], a standard in situ 96-well sitting-drop crystallization plate was used. The plate with the sample was left for several days until crystals grew to their maximum size. Instead of harvesting and mounting the crystals, the team mounted the entire plate on the beamline and standard procedures were then used for data collection from the membrane protein crystals. Results demonstrated that membrane protein structures can be determined at a synchrotron source using in situ room-temperature data-collection strategies.
Huang et al. [Acta Cryst. (2015). D71, 1238-1256; doi:10.1107/S1399004715005210] took the in situ approach one stage further and showed the applicability of room-temperature data collection for in meso/in situ crystallization and its use for high-throughput crystallography of membrane proteins crystallized in meso using LCP technology.
The two in situ high-throughput methodologies open up new perspectives in X-ray crystallography of membrane proteins and will provide a more rapid route to structure determination where the crystals are too small or fragile to mount, or where radiation sensitivity requires data collection from hundreds of crystals. In situ data collection therefore provides an excellent alternative to data collection at the X-ray free-electron laser, which cannot currently provide sufficient time for users.


